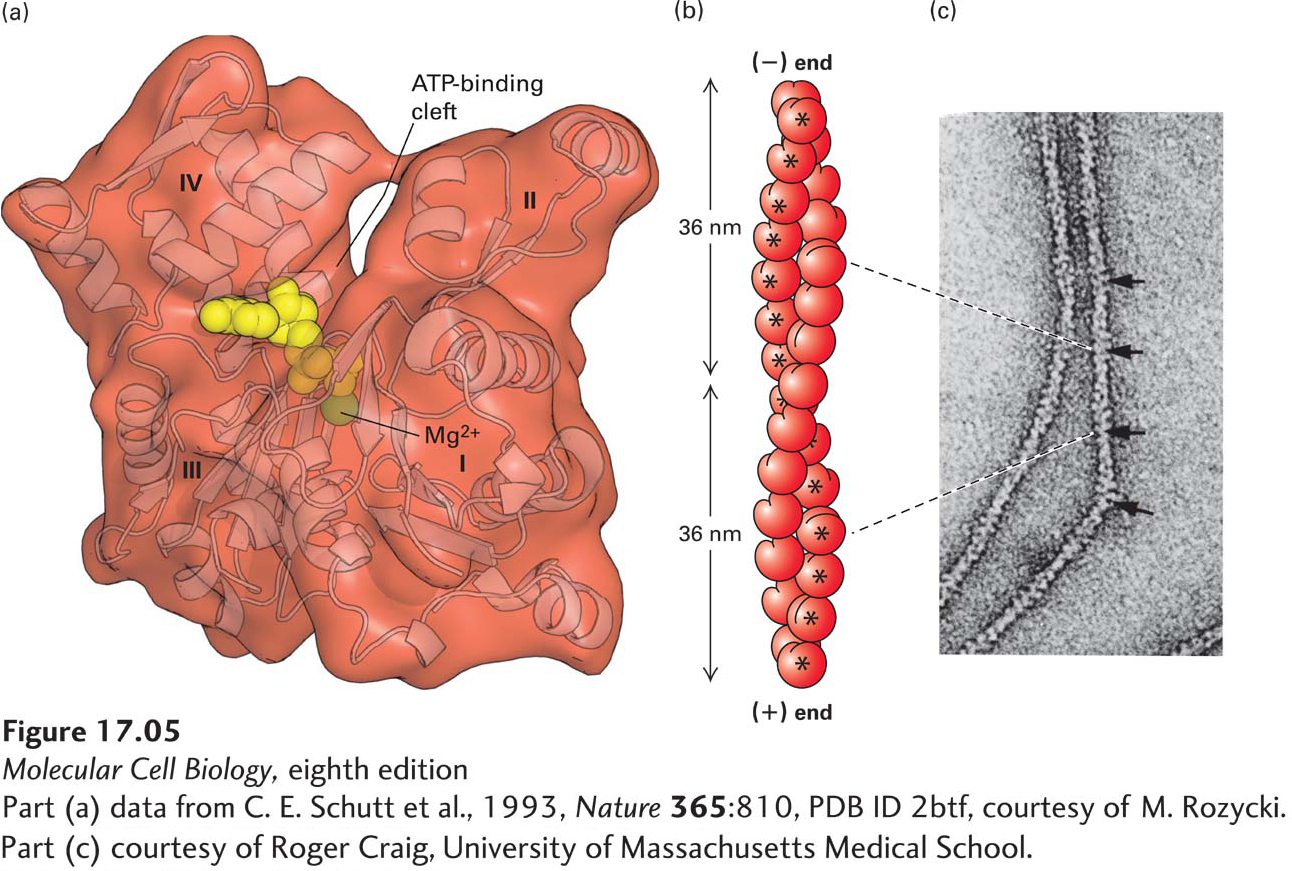
FIGURE 17- 5 Structures of monomeric G- actin and F- actin filaments. (a) Structure of the actin monomer (measuring 5.5 × 5.5 × 3.5 nm), which is divided by a central cleft into two approximately equal- sized lobes and four subdomains, numbered I– IV. ATP (red) binds at the bottom of the cleft and contacts both lobes (the yellow ball represents Mg2+). The N- and C- termini lie in subdomain I. (b) An actin filament appears as two strands of subunits. One repeating unit consists of 28 subunits (14 in each strand, indicated by * for one strand), covering a distance of 72 nm. The ATP- binding cleft of every actin subunit is oriented toward the same end of the filament. The end of a filament with an exposed binding cleft is the (−) end; the opposite end is the (+) end. (c) In the electron microscope, negatively stained actin filaments appear as long, flexible, and twisted strands of beaded subunits. Because of the twist, the filament appears alternately thinner (7- nm diameter) and thicker (9- nm diameter) (arrows). (The microfilaments visualized in a cell by electron microscopy are F- actin filaments plus any bound proteins.)
[Part (a) data from C. E. Schutt et al., 1993, Nature 365:810, PDB ID 2btf, courtesy of M. Rozycki. Part (c) courtesy of Roger Craig, University of Massachusetts Medical School.]
[Leave] [Close]