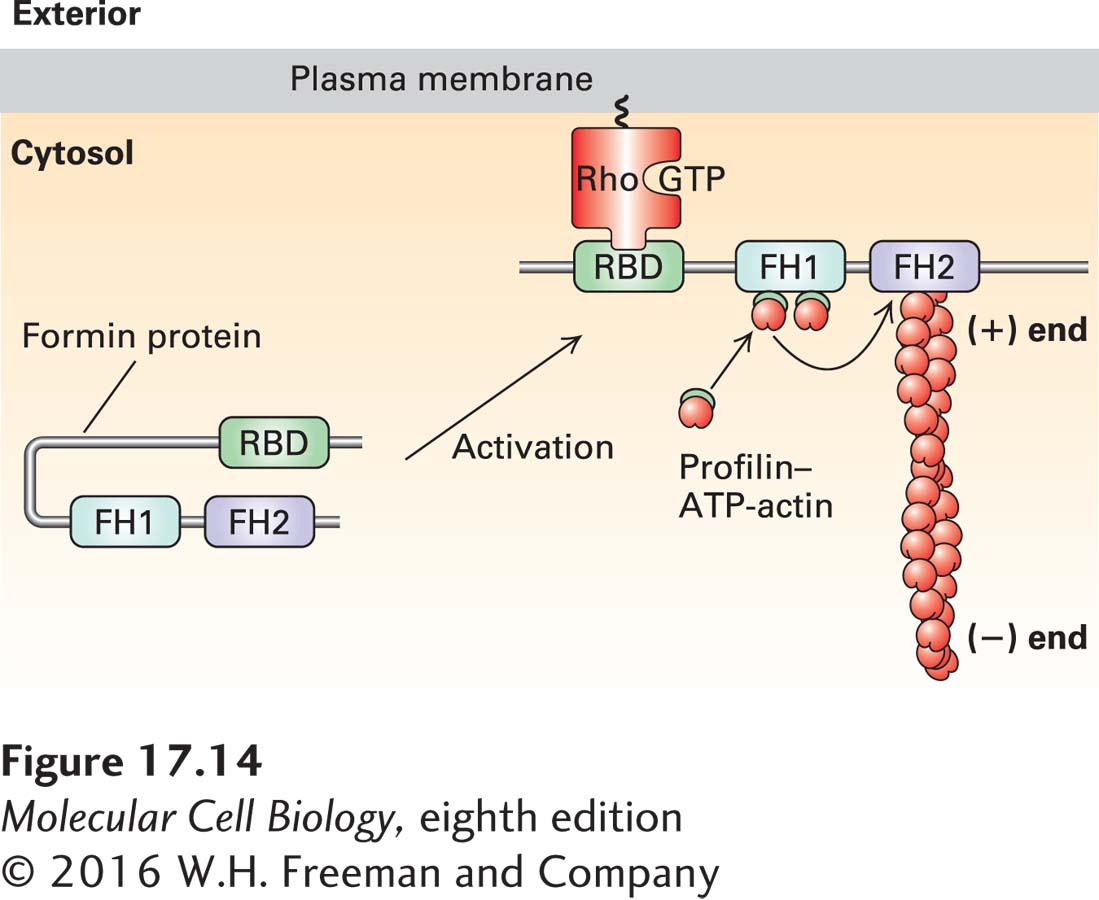
FIGURE 17- 14 Regulation of formin activity by an intramolecular interaction. Some of the formin classes found in vertebrates are regulated by an intramolecular interaction. In its inactive state, the formin folds back on itself to inhibit the activity of the FH2 domain. The inactive formin is activated when its Rho- binding domain (RBD) binds to membrane- bound active Rho- GTP, resulting in exposure of the formin’s FH2 domain, which can then nucleate the assembly of a new actin filament. All formins have an FH1 domain adjacent to the FH2 domain; the proline- rich FH1 domain is a site for recruitment of profilin– ATP– G- actin complexes that can then be “fed” into the growing (+) end. For simplicity of representation, a single formin protein is shown, but as shown in Figure 17- 13 , the FH2 domain functions as a dimer to nucleate actin assembly. Regulation of the Rho family of small GTPases is detailed in Figures 17- 41 and 17- 43 .
[Leave] [Close]