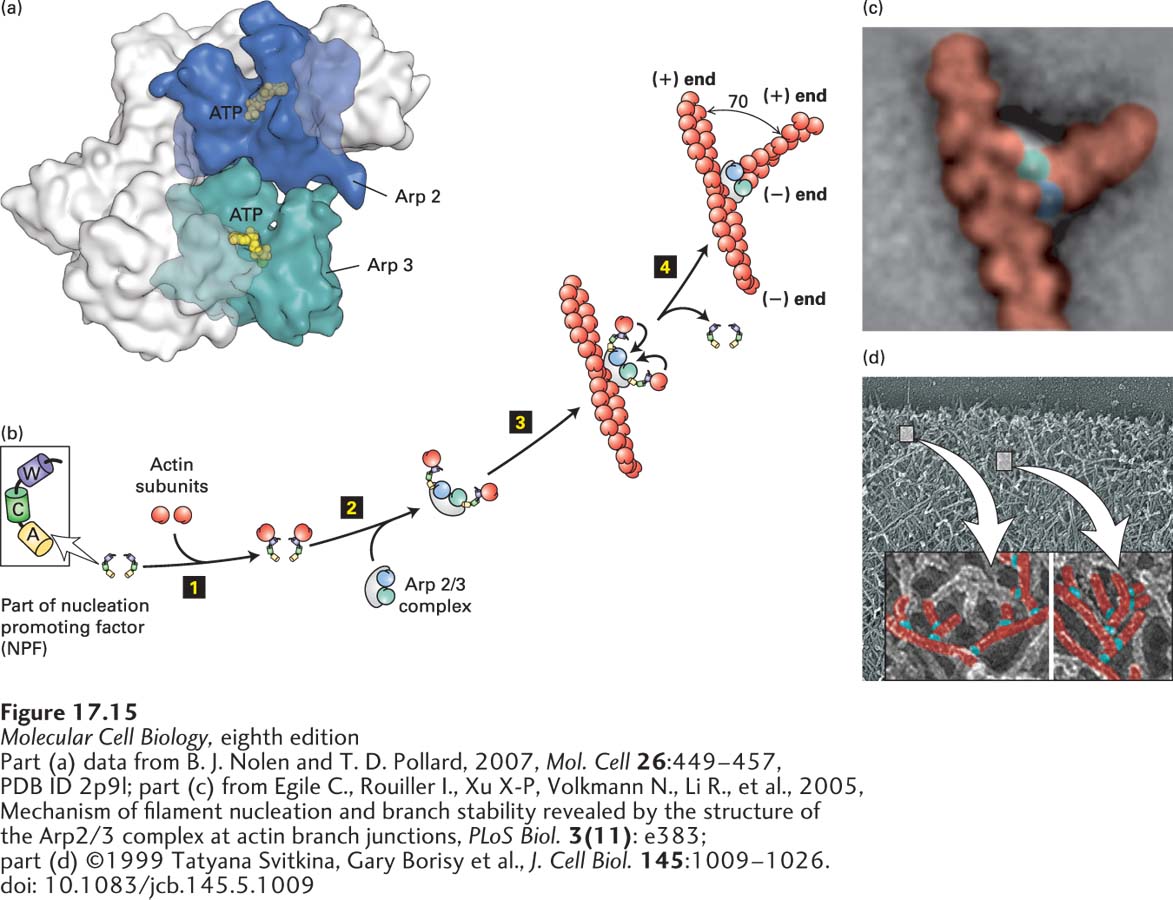
FIGURE 17- 15 Actin nucleation by the Arp2/3 complex. (a) X- ray crystallographic structure of the Arp2/3 complex, with five of the subunits in gray and the Arp2 and Arp3 subunits in green and blue. (b) To nucleate actin assembly efficiently, Arp2/3 must interact with the activating part of an NPF, shown here with its W (WH2), C (connector), and A (acidic) domains. The first step 1 involves binding of an actin subunit to the W domain of each NPF. Two NPF- actin complexes then bind the Arp2/3 complex step 2. This interaction induces a conformational change in the Arp2/3 complex. After binding of the Arp2/3 complex to the side of an actin filament, the actin subunits delivered by the W domains bind to the Arp2/3 complex step 3, which then initiates the assembly of an actin filament at the available (+) end step 4. The Arp2/3 branch makes a characteristic 70° angle between the old and new filaments. (c) Averaged image compiled from several electron micrographs of Arp2/3 at an actin branch. (d) Image of actin filaments in the leading edge of a motile cell, with a magnification and coloring of individual branched filaments.
[Part (a) data from B. J. Nolen and T. D. Pollard, 2007, Mol. Cell 26:449– 457, PDB ID 2p9l; part (c) from Egile C., Rouiller I., Xu X- P, Volkmann N., Li R., et al., 2005, Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions, PLoS Biol. 3(11): e383; part (d) ©1999 Tatyana Svitkina, Gary Borisy et al., J. Cell Biol. 145:1009– 1026. doi: 10.1083/jcb.145.5.1009]
[Leave] [Close]