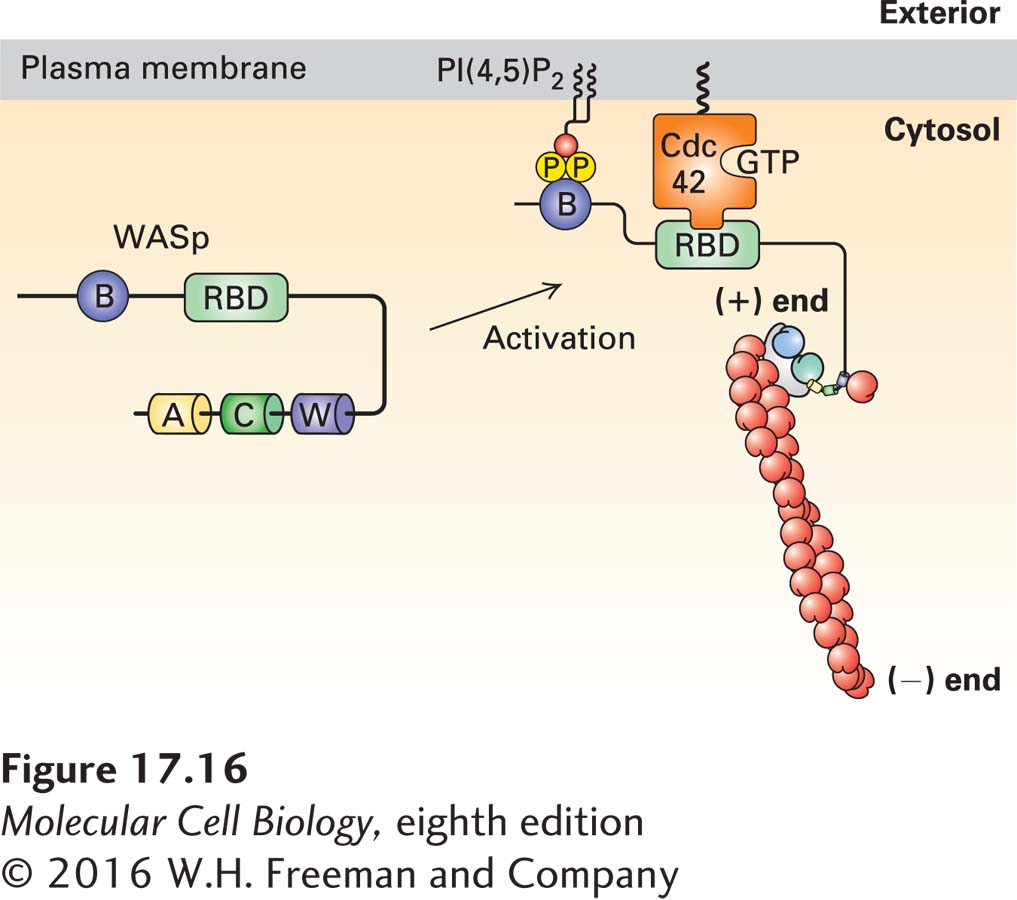
FIGURE 17- 16 Regulation of the Arp2/3 complex by WASp and PI(4,5)P2. The NPF WASp is inactive due to an intramolecular interaction that masks the WCA domain. It is activated by a coincidence detection mechanism: it must bind both the regulatory phospholipid PI(4,5)P2 though its basic domain (B) and the membrane- bound active small G protein Cdc42- GTP (a member of the Rho family) through its Rho- binding domain (RBD). When activated in this way, the intramolecular interaction in WASp is relieved, allowing the W domain to bind actin and the acidic A domain to activate the Arp2/3 complex. For simplicity, only a single NFP- Arp2/3 interaction is shown. Regulation of the Rho family of small GTPases is detailed in Figures 17- 41 and 17- 43 .
[Leave] [Close]