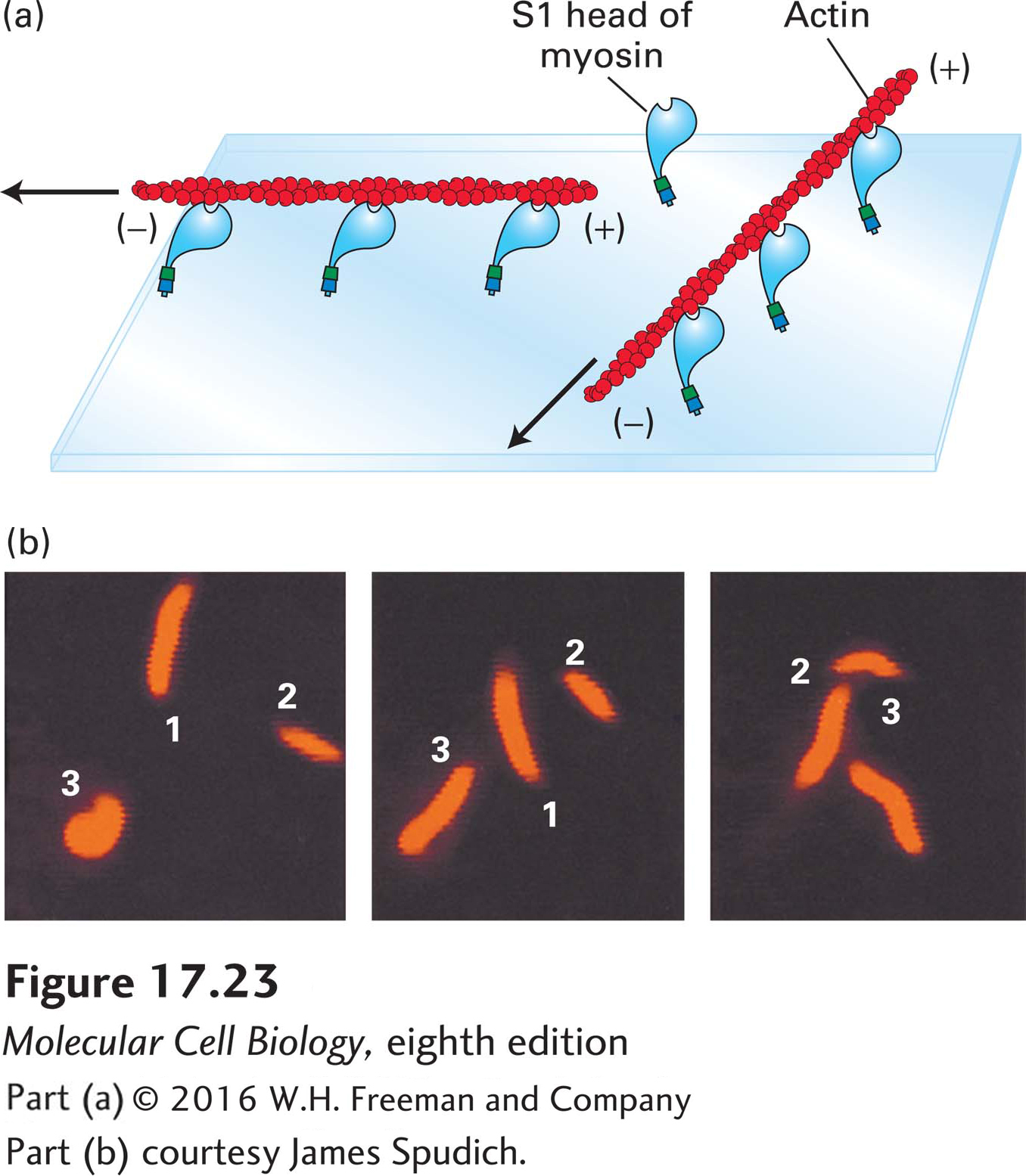
EXPERIMENTAL FIGURE 17- 23 The sliding- filament assay is used to detect myosin- powered movement. (a) After myosin molecules are adsorbed onto the surface of a glass coverslip, excess unbound myosin is removed; the coverslip is then placed myosin- side- down on a glass slide to form a chamber through which solutions can flow. A solution of actin filaments, made visible and stable by staining with rhodamine- labeled phalloidin, is allowed to flow into the chamber. In the presence of ATP, the myosin heads “walk” toward the (+) ends of the actin filaments by the mechanism discussed later and illustrated in Figure 17- 26 . Because the myosin tails are immobilized, walking of the heads toward the (+) ends causes sliding of the filaments, which appear to be moving with their (−) ends leading the way. Movement of individual filaments can be observed in a fluorescence light microscope. (b) These photographs show the positions of three actin filaments (numbered 1, 2, 3) at 30- second intervals recorded by video microscopy. The rate of filament movement can be determined from such recordings.
[Part (b) courtesy James Spudich.]
[Leave] [Close]