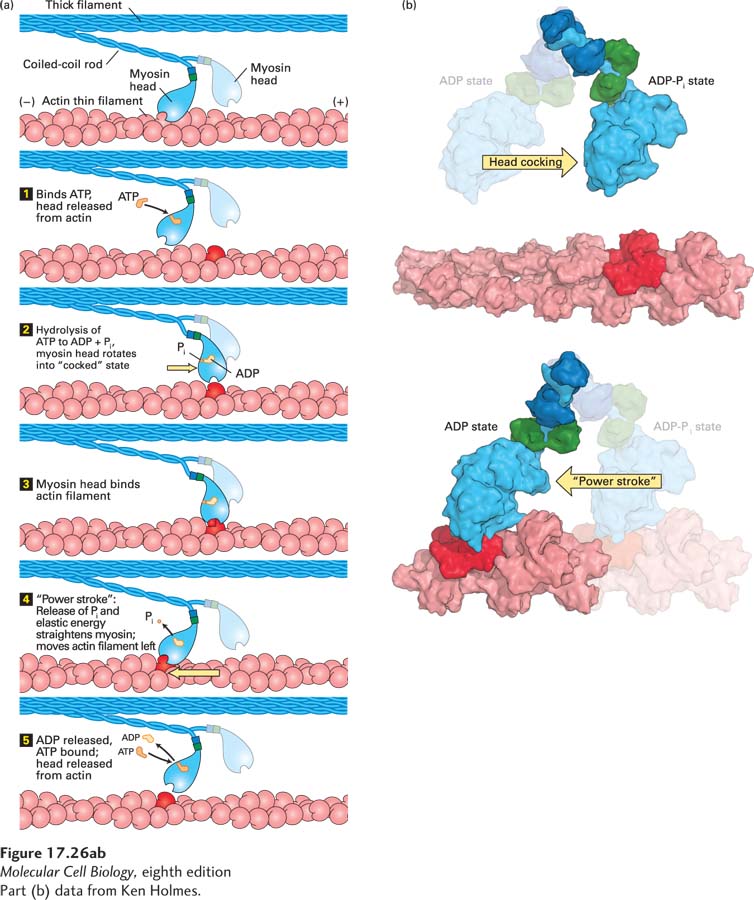
FIGURE 17- 26 ATP- driven myosin movement along actin filaments. (a) In the absence of ATP, the myosin head is firmly attached to the actin filament. Although this state is very short- lived in living muscle, it is the state responsible for muscle stiffness in death (rigor mortis). Step 1: On binding ATP, the myosin head releases from the actin filament. Step 2: The head hydrolyzes the ATP to ADP and Pi, which induces a rotation in the head with respect to the neck. This “cocked state” stores the energy released by ATP hydrolysis as elastic energy, like a stretched spring. Step 3: Myosin in the “cocked” state is stable until it binds actin. Step 4: When it is bound to actin, the myosin head couples release of Pi with release of the elastic energy to move the actin filament. This movement is known as the “power stroke,” as it involves moving the actin filament with respect to the end of the myosin neck domain. Step 5: The head remains tightly bound to the actin filament until ADP is released and fresh ATP is bound by the head. See R. D. Vale and R. A. Milligan, 2002, Science 288:88. (b) Molecular models of the conformational changes in the myosin head involved in “cocking” the head (upper panel) and after the power stroke (lower panel). The myosin light chains are shown in dark blue and green; the rest of the myosin head and neck are colored light blue, and actin is red. See S. Fischer et al., 2005, Proc. Natl. Acad. Sci. USA 102:6873– 6876.
[Part (b) data from Ken Holmes.]
[Leave] [Close]