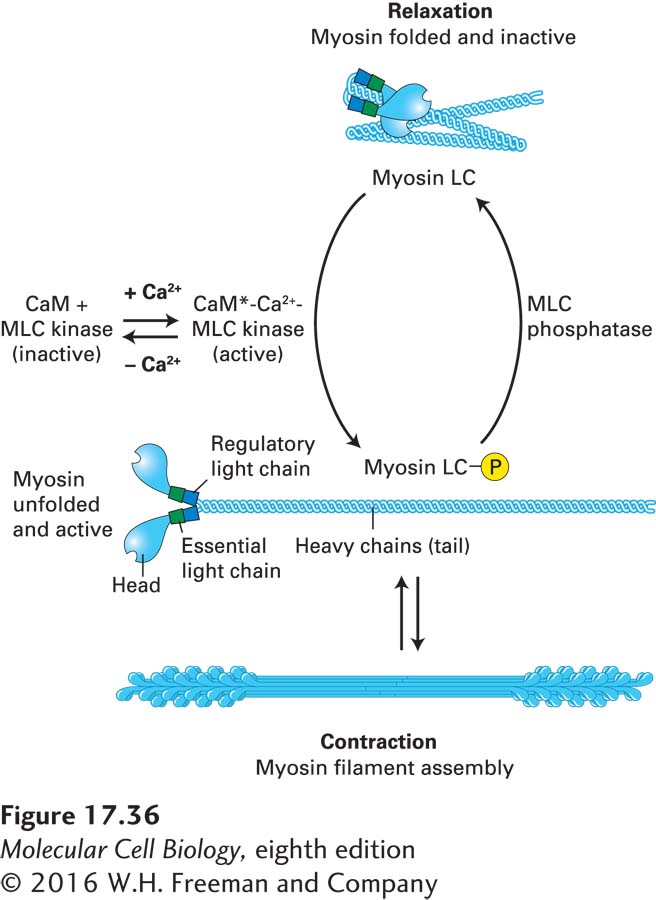
FIGURE 17- 36 Myosin light- chain phosphorylation regulates smooth muscle contraction. In vertebrate smooth muscle, phosphorylation of the myosin regulatory light chain (LC) activates contraction. At Ca2+ concentrations of less than 10−6 M, the regulatory light chain is not phosphorylated, and the myosin adopts a folded conformation. When the Ca2+ level rises, Ca2+ binds calmodulin (CaM), which undergoes a conformational change (CaM*). The CaM*-Ca2+ complex binds and activates myosin light- chain kinase (MLC kinase), which then phosphorylates the myosin LC. This phosphorylation event unfolds the myosin II, which is now active and can assemble into bipolar filaments to participate in contraction. When the Ca2+ levels drop, the myosin LC is dephosphorylated by myosin light- chain (MLC) phosphatase, which is not dependent on Ca2+ for activity, causing muscle relaxation.
[Leave] [Close]