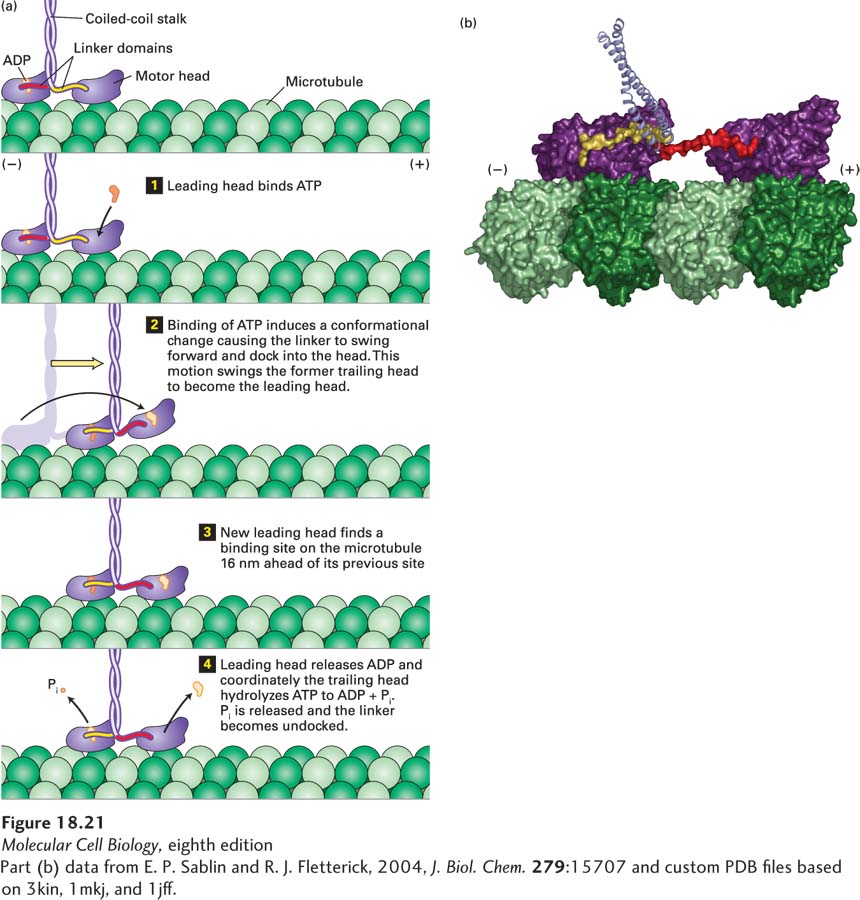
FIGURE 18- n-
[Part (b) data from E. P. Sablin and R. J. Fletterick, 2004, J. Biol. Chem. 279:15707 and custom PDB files based on 3kin, 1mkj, and 1jff.]