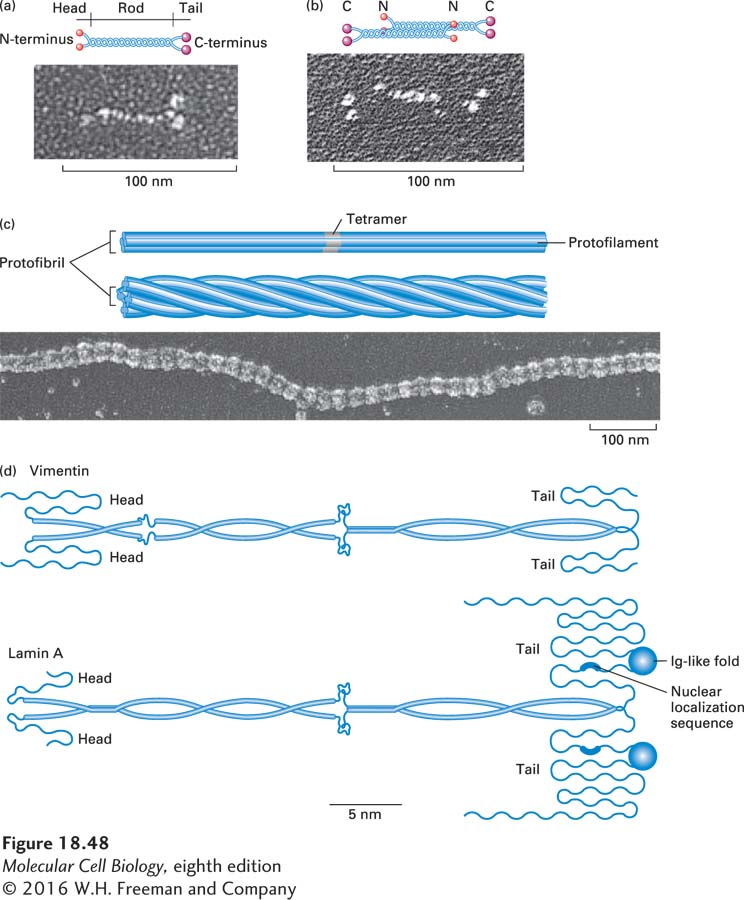
FIGURE 18- d- e- y- n-