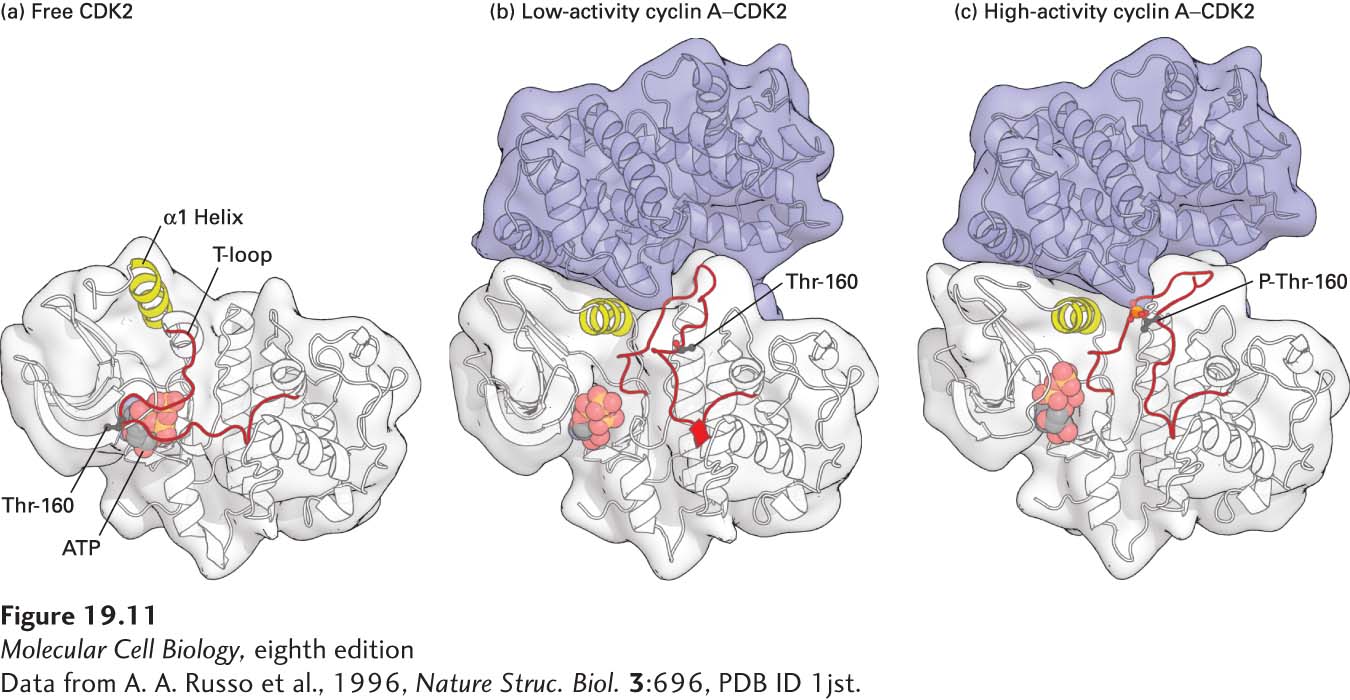
FIGURE 19- 11 Structural models of human CDK2. (a) Free, inactive CDK2 not bound to its cyclin subunit, cyclin A. In free CDK2, the T loop blocks access of protein substrates to the γ phosphate of the bound ATP, shown as a ball- and- stick model. The conformations of the T- loop and the region highlighted in yellow (α1 Helix) are altered when CDK is bound to cyclin A. (b) Nonphosphorylated, low- activity cyclin A– CDK2 complex. Conformational changes induced by binding of a domain of cyclin A (blue) cause the T loop to pull away from the active site of CDK2 so that substrate proteins can bind. The α1 helix in CDK2, which interacts extensively with cyclin A, moves several angstroms into the catalytic cleft, repositioning key catalytic side chains required for the phosphotransfer reaction. The black ball marks the position of the threonine (Thr- 160) whose phosphorylation activates CDKs. (c) Phosphorylated, high- activity cyclin A– CDK2 complex. The conformational changes induced by phosphorylation of the activating threonine (red ball) alter the shape of the substrate- binding surface, greatly increasing the affinity for protein substrates. See P. D. Jeffrey et al., 1995, Nature 1995, 376:313- 20.
[Data from A. A. Russo et al., 1996, Nature Struc. Biol. 3:696, PDB ID 1jst.]
[Leave] [Close]