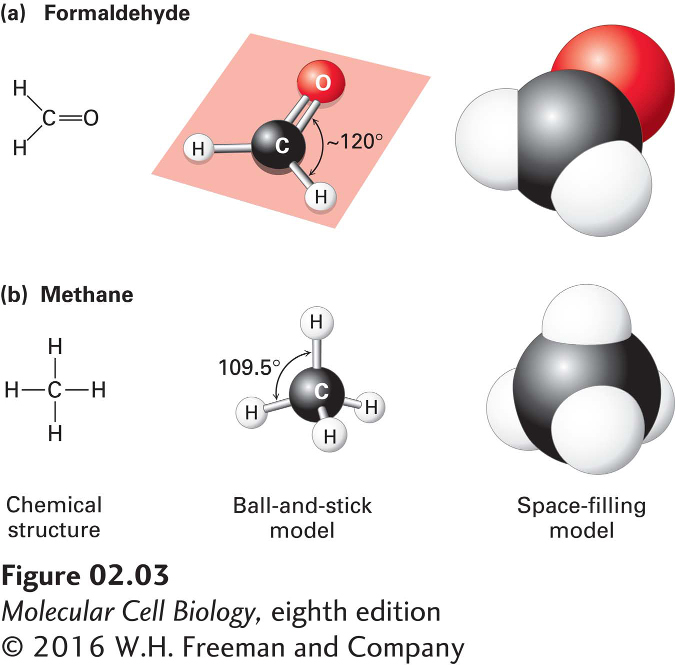
FIGURE 2- 3 Geometry of bonds when carbon is covalently linked to three or four other atoms. (a) A carbon atom can be bonded to three atoms, as in formaldehyde (CH2O). In this case, the carbon- bonding electrons participate in two single bonds and one double bond, which all lie in the same plane. Unlike atoms connected by a single bond, which usually can rotate freely about the bond axis, those connected by a double bond cannot. (b) When a carbon atom forms four single bonds, as in methane (CH4), the bonded atoms (all H in this case) are oriented in space in the form of a tetrahedron. The letter representations on the left clearly indicate the atomic composition of each molecule and its bonding pattern. The ball- and- stick models in the center illustrate the geometric arrangement of the atoms and bonds, but the diameters of the balls representing the atoms and their nonbonding electrons are unrealistically small compared with the bond lengths. The sizes of the electron clouds in the space- filling models on the right more accurately represent the structure in three dimensions.
[Leave] [Close]