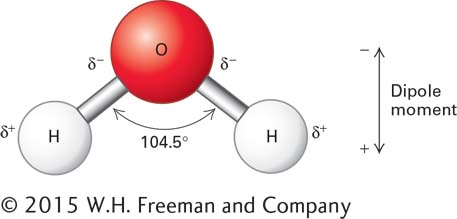
FIGURE 2- 5 The dipole nature of a water molecule. The symbol δ represents a partial charge (a weaker charge than the one on an electron or a proton). Because of the difference in the electronegativities of H and O, each of the polar H– O bonds in water is a dipole. The sizes and directions of the dipoles of each of the bonds determine the net distance and amount of charge separation, or dipole moment, of the molecule.
[Leave] [Close]