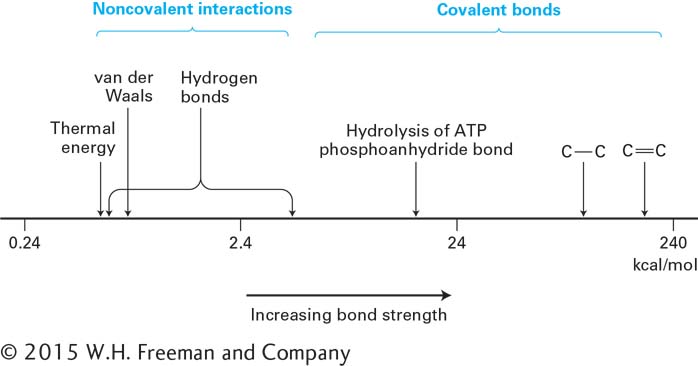
FIGURE 2- C– n-