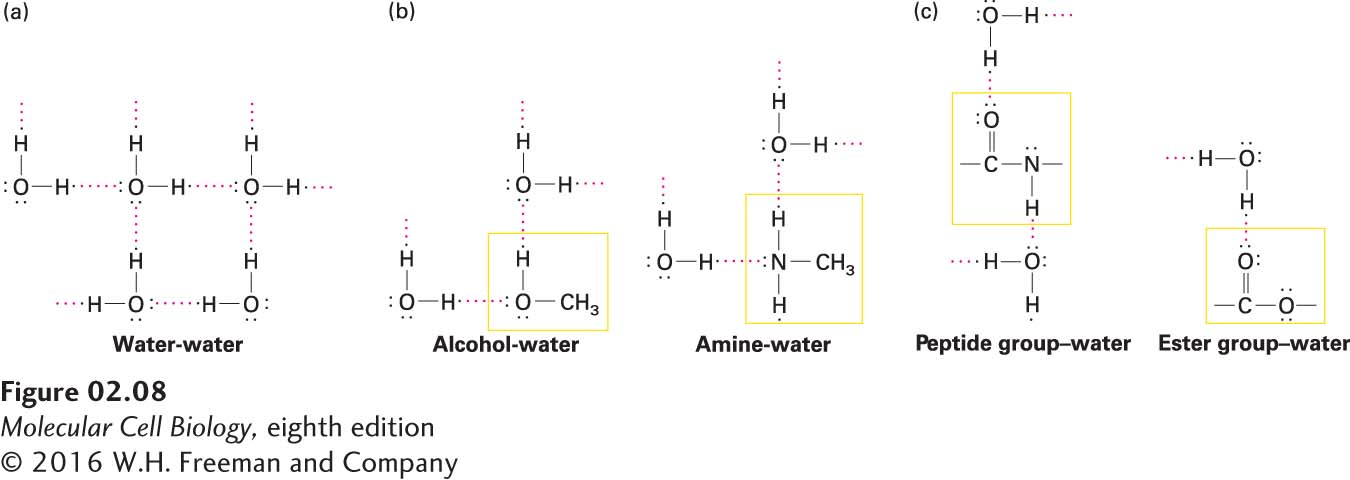
FIGURE 2- 8 Hydrogen bonding of water with itself and with other compounds. Each pair of nonbonding outer electrons in an oxygen or a nitrogen atom can accept a hydrogen atom in a hydrogen bond. The hydroxyl and the amino groups can also form hydrogen bonds with water. (a) In liquid water, each water molecule forms transient hydrogen bonds with several others, creating a dynamic network of hydrogen- bonded molecules. (b) Water can also form hydrogen bonds with alcohols and amines, which accounts for the high solubility of these compounds. (c) The peptide group and the ester group, which are present in many biomolecules, commonly participate in hydrogen bonds with water or polar groups in other molecules.
[Leave] [Close]