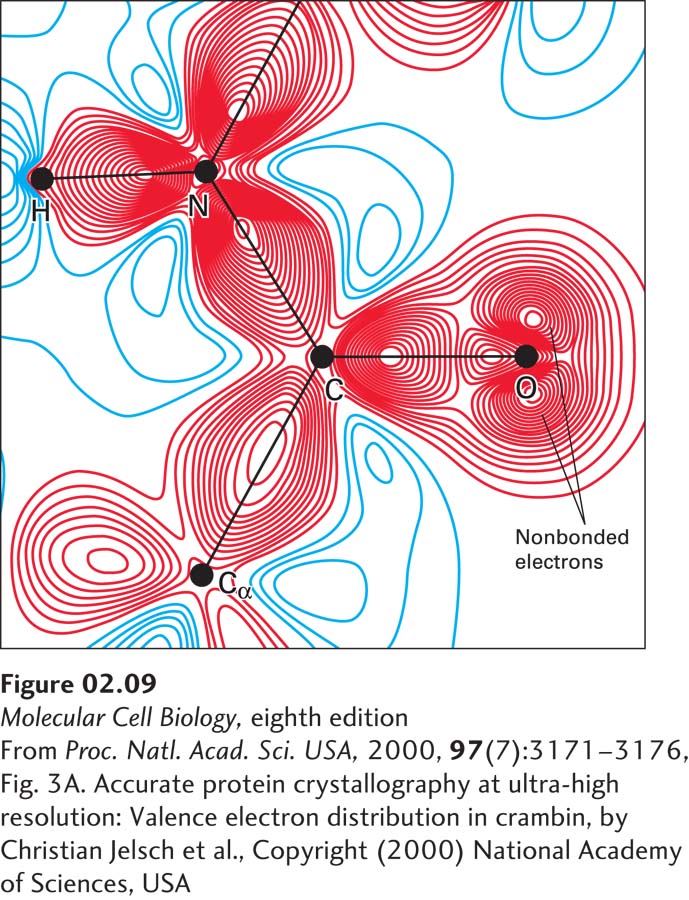
FIGURE 2- 9 Distribution of bonding and outer nonbonding electrons in the peptide group. Shown here is a peptide bond linking two amino acids within a protein called crambin. No protein has been structurally characterized at higher resolution than crambin. The black lines represent the covalent bonds between atoms. The red (negative) and blue (positive) lines represent contours of charge determined using x- ray crystallography and computational methods. The greater the number of contour lines, the higher the charge. The high density of red contour lines between atoms represents the covalent bonds (shared electron pairs). The two sets of red contour lines emanating from the oxygen (O) and not falling on a covalent bond (black line) represent the two pairs of nonbonding electrons on the oxygen that are available to participate in hydrogen bonding. The high density of blue contour lines near the hydrogen (H) bonded to nitrogen (N) represents a partial positive charge, indicating that this H can act as a donor in hydrogen bonding.
[From Proc. Natl. Acad. Sci. USA, 2000, 97(7):3171– 3176, Fig. 3A. Accurate protein crystallography at ultra- high resolution: Valence electron distribution in crambin, by Christian Jelsch et al., Copyright (2000) National Academy of Sciences, USA.]
[Leave] [Close]