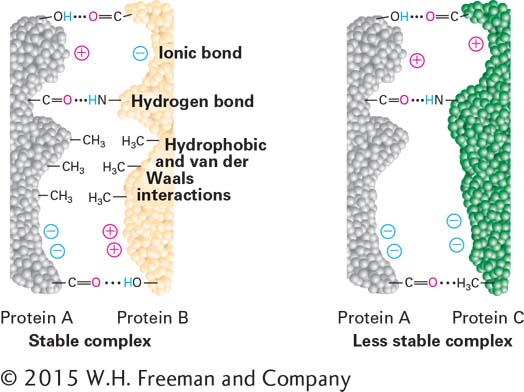
FIGURE 2- 12 Molecular complementarity permits tight protein bonding via multiple noncovalent interactions. The complementary shapes, charges, polarity, and hydrophobicity of two protein surfaces permit multiple weak interactions, which in combination produce a strong interaction and tight binding. Because deviations from molecular complementarity substantially weaken binding, a particular surface region of any given biomolecule usually can bind tightly to only one or a very limited number of other molecules. The complementarity of the two protein molecules on the left permits them to bind much more tightly than the two noncomplementary proteins on the right.
[Leave] [Close]