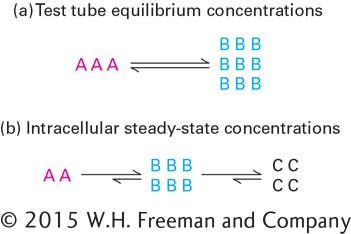
FIGURE 2- d—