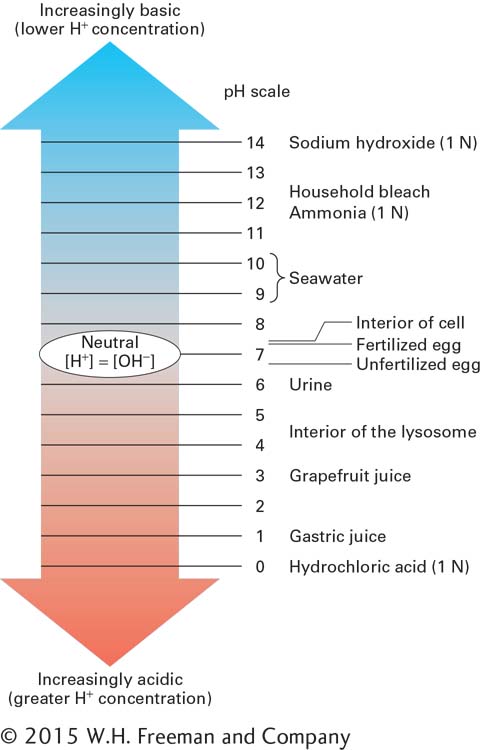
FIGURE 2- 25 Some pH values for common solutions. The pH of an aqueous solution is the negative log of the hydrogen ion concentration. The pH values for most intracellular and extracellular biological fluids are near 7 and are carefully regulated to permit the proper functioning of cells, organelles, and cellular secretions. The pH values for solutions of ammonia and hydrochloric acid are for one normal (1 N) solutions.
[Leave] [Close]