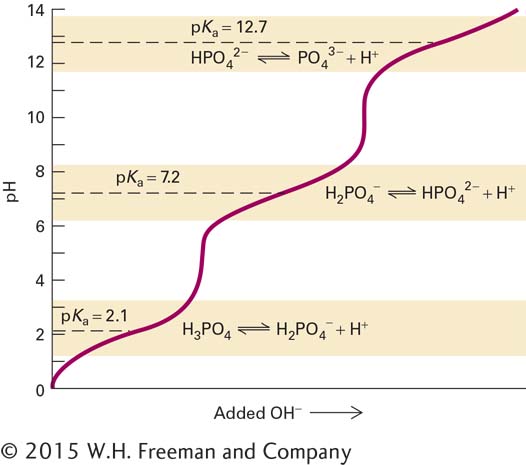
FIGURE 2- s— s—