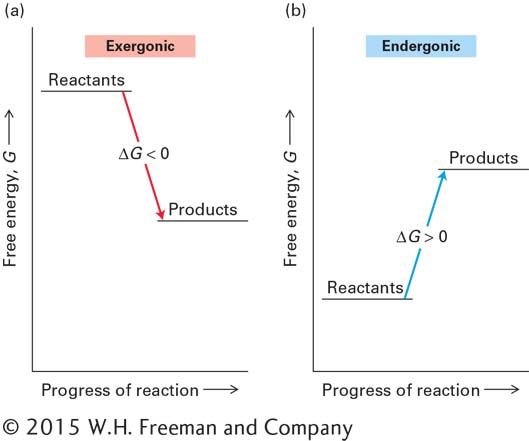
FIGURE 2- 29 Changes in the free energy (ΔG) of exergonic and endergonic reactions. (a) In exergonic reactions, the free energy of the products is less than that of the reactants. Consequently, these reactions occur spontaneously, and energy is released as the reactions proceed. (b) In endergonic reactions, the free energy of the products is greater than that of the reactants, and these reactions do not occur spontaneously. An external source of energy must be supplied if the reactants are to be converted into products.
[Leave] [Close]