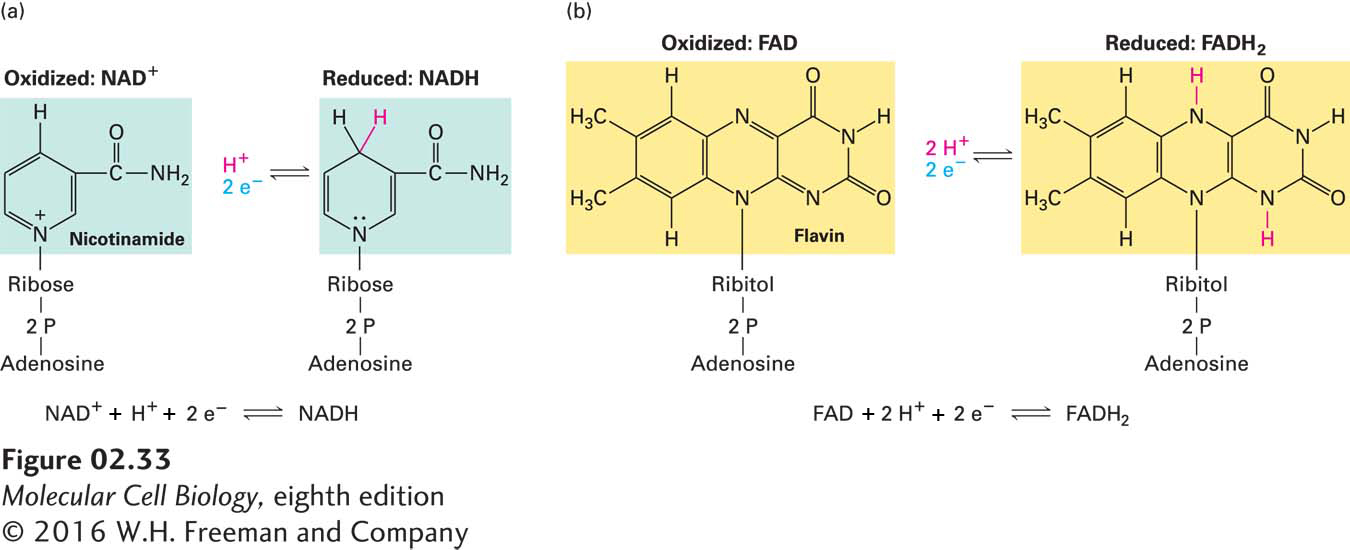
FIGURE 2- 33 The electron- carrying coenzymes NAD + and FAD. (a) NAD+ (nicotinamide adenine dinucleotide) is reduced to NADH by the addition of two electrons and one proton simultaneously. In many biological redox reactions, a pair of hydrogen atoms (two protons and two electrons) is removed from a molecule. In some cases, one of the protons and both electrons are transferred to NAD+; the other proton is released into solution. (b) FAD (flavin adenine dinucleotide) is reduced to FADH2 by the addition of two electrons and two protons, as occurs when succinate is converted to fumarate (see Figure 2- 32 ). In this two- step reaction, addition of one electron together with one proton first generates a short- lived semiquinone intermediate (not shown), which then accepts a second electron and proton.
[Leave] [Close]