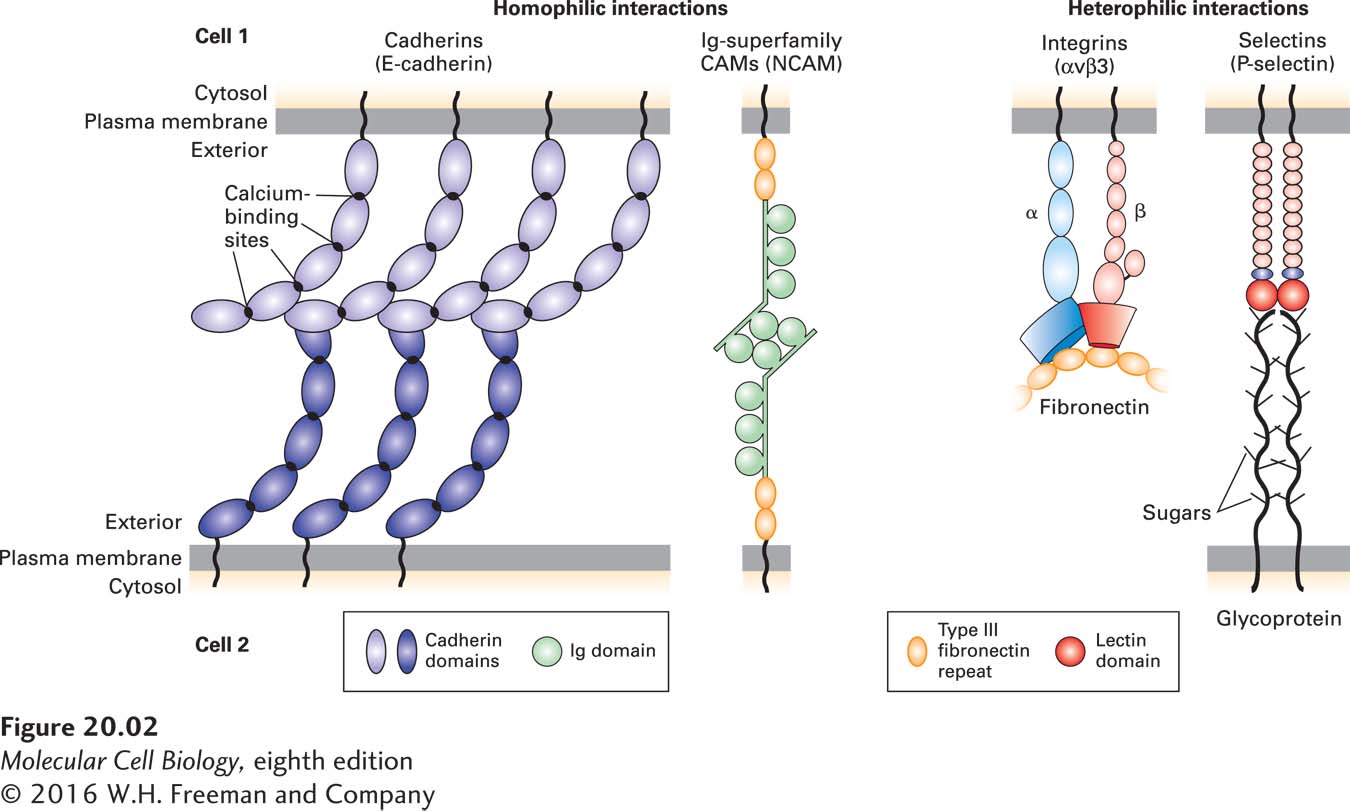
FIGURE 20- 2 Major families of cell- adhesion molecules (CAMs) and adhesion receptors. E- cadherins commonly form cross- bridges with other E- cadherins (homophilic binding) on the same cell or on adjacent cells (see Figures 20- 3 and 20- 14 ). Members of the immunoglobulin (Ig) superfamily of CAMs can function as adhesion receptors or as CAMs that form homophilic linkages (as shown here for NCAM) or heterophilic linkages (to other types of CAMs, not shown). Heterodimeric integrins (for example, αv and β3 chains) function as CAMs or as adhesion receptors (shown here) that bind to very large, multi- adhesive matrix proteins such as fibronectin, only a small part of which is shown here. Selectins, shown as dimers, contain a carbohydrate- binding lectin domain that recognizes specialized sugar structures on glycoproteins (as shown here) or glycolipids on adjacent cells. Note that CAMs often form higher- order oligomers within the plane of the plasma membrane. Many adhesion molecules contain multiple distinct domains, some of which are found in more than one kind of CAM. The cytoplasmic domains of these proteins are often associated with adapter proteins that link them to the cytoskeleton or to signaling pathways. See R. O. Hynes, 1999, Trends Cell Biol. 9:M33, R. O. Hynes, 2002, Cell 110:673– 687, and J. Brasch, O. J. Harrison, B. Honig, and L. Shapiro, 2012, Trends Cell Biol. 22:299– 310.
[Leave] [Close]