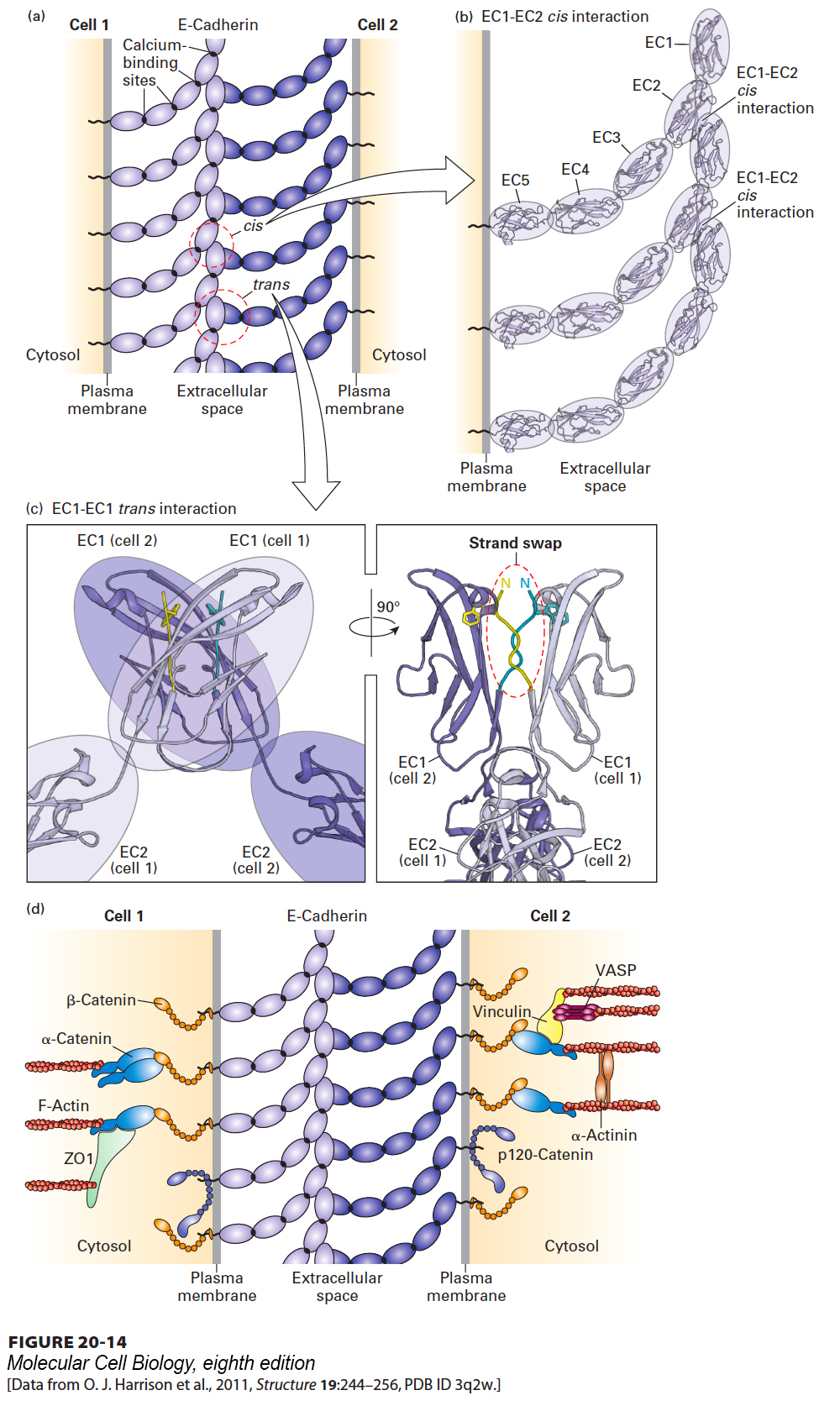
FIGURE 20- 14 Intercellular and intracellular interactions of classical cadherins in typical adherens junctions. (a)The exoplasmic cadherin domains [EC1- EC5, see ovals in part (b)] of E- cadherins at adherens junctions on adjacent cells are clustered by homophilic cis and trans interactions. The Ca2+-dependent elongated and curved structure of cadherin’s extracellular domains is necessary for stable cis and trans interactions. Sites representing individual cis and trans interactions are highlighted by dashed circles. (b) EC1- EC2 cis interaction: The binding of an EC1 domain of one cadherin to an EC2 domain of an adjacent cadherin on the same cell is responsible for cis interactions. In panels (b) and (c) the structure of each extracellular cadherin domain determined by X- ray crystallography is represented using a ribbon diagram and is highlighted by an oval. (c) EC1- EC1 trans interaction: Two views rotated by 90° of the trans binding of an EC1 domain of one cadherin to an EC1 domain of a cadherin on the adjacent cell. Only the EC1 and a portion of the EC2 domains of two trans interacting cadherins are shown. The left view shows the relative orientations of the main axes of the oval- shaped EC1 domains. The right view shows how a small segment of polypeptide at the N- terminus of each of the two EC1 domains [highlighted in yellow (cell 1) and blue (cell 2)] swings out and replaces the equivalent segment from its binding partner (strand swap, dashed oval). The strand swap places the side chain of a tryptophan residue on each of the segments into a binding pocket on the adjacent EC1 domain – an interaction that substantially stabilizes the trans binding. (d) The cytosolic domains of the E- cadherins bind directly or indirectly to multiple adapter proteins (e.g., β-catenin), which both connect the junctions to actin filaments (F- actin) of the cytoskeleton and participate in intracellular signaling pathways. Somewhat different sets of adapter proteins are illustrated in the two cells to emphasize that a variety of adapters can interact with adherens junctions. Some of these adapters, such as ZO- 1, can interact with several different CAMs. See V. Vasioukhin and E. Fuchs, 2001, Curr. Opin. Cell Biol. 13:76 and J. Brasch, O. J. Harrison, B. Honig, and L. Shapiro, 2012, Trends Cell Biol. 22:299.
[Data from O. J. Harrison et al., 2011, Structure 19:244– 256, PDB ID 3q2w.]
[Leave] [Close]