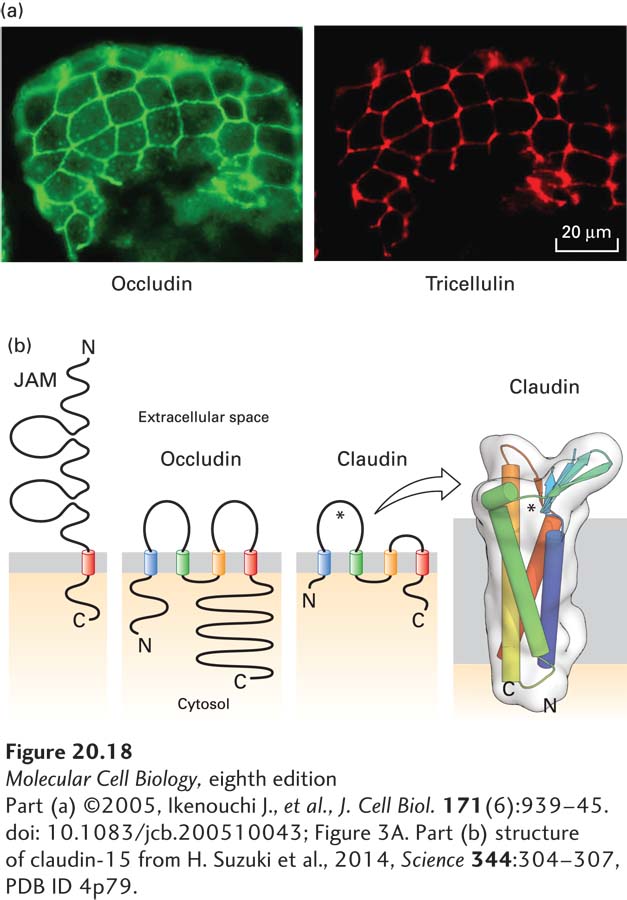
FIGURE 20- 18 Proteins that compose tight junctions. (a) Immunofluorescence localization of occludin (green) and tricellulin (red) in mouse intestinal epithelium. Note that tricellulin is predominantly concentrated in tricellular junctions. (b) The junction adhesion molecule (JAM) has a single transmembrane domain and an extracellular region with two immunoglobulin domains, whereas occludin and claudins contain four transmembrane helices. The larger extracellular loop of the claudins, indicated by an asterisk, contributes to paracellular ion selectivity. The transmembrane helices of claudin- 15, which permits paracellular transport of cations, form a four- helix bundle, and the extracellular loops contain a five- stranded β sheet (seen edgewise in this view). This β sheet has been proposed to help define the pore through which ions pass (near the asterisk). See S. Tsukita et al., 2001, Nat. Rev. Mol. Cell Biol. 2:285.
[Part (a) ©2005, Ikenouchi J., et al., J. Cell Biol. 171(6):939– 45. doi: 10.1083/jcb.200510043; Figure 3A. Part (b) structure of claudin- 15 from H. Suzuki et al., 2014, Science 344:304– 307, PDB ID 4p79.]
[Leave] [Close]