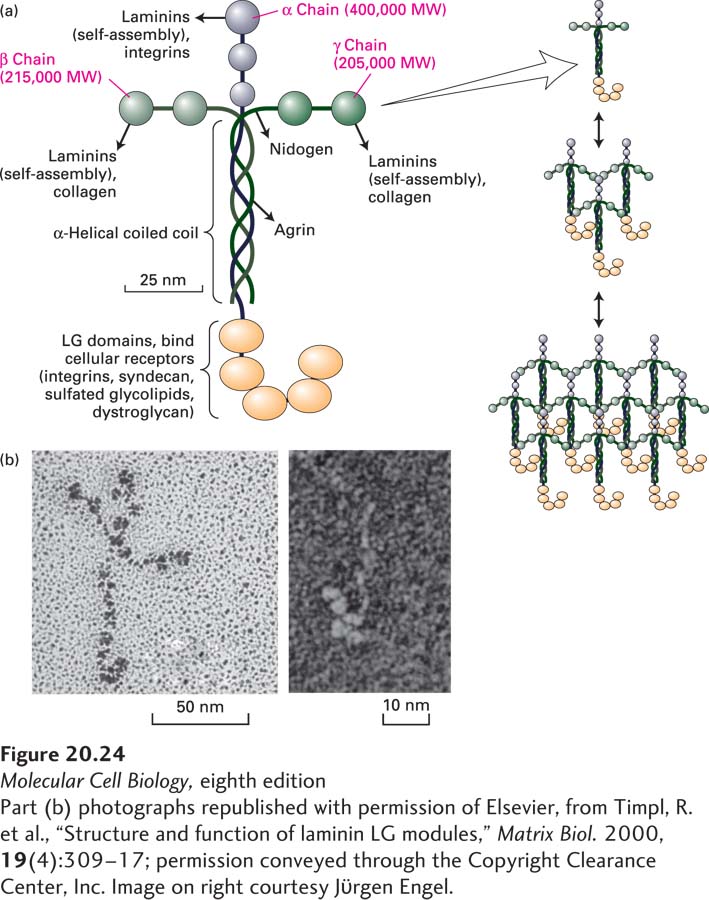
FIGURE 20- 24 Laminin is a heterotrimeric multi- adhesive matrix protein found in all basal laminae. (a) Schematic model of cross- shaped laminin molecule showing the general shape, location of globular domains, and coiled- coil region in which laminin’s three chains are covalently linked by several disulfide bonds. Different regions of laminin bind to adhesion receptors and various matrix components (indicated by arrows). Right: Laminins assemble into a lattice via interactions between their N- terminal globular domains. See G. R. Martin and R. Timpl, 1987, Annu. Rev. Cell Biol. 3:57; M. Durbeej, 2010, Cell Tissue Res. 339:259– 268; and S. Meinen et al., 2007, J. Cell Biol. 176:979– 993. (b) Electron micrographs of an intact laminin molecule, showing its characteristic cross shape (left), and the carbohydrate- binding LG domains near the C- terminus (right).
[Part (b) photographs republished with permission of Elsevier, from Timpl, R. et al., “Structure and function of laminin LG modules,” Matrix Biol. 2000, 19(4):309– 17; permission conveyed through the Copyright Clearance Center, Inc. Image on right courtesy Jϋrgen Engel.]
[Leave] [Close]