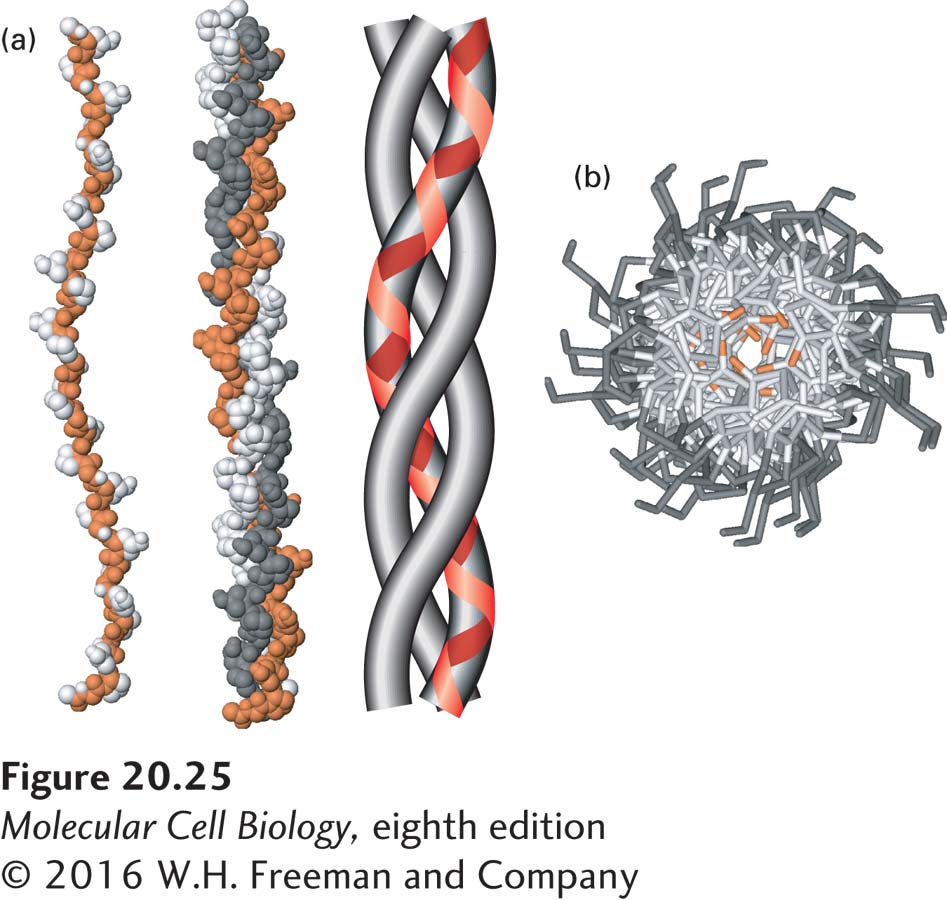
FIGURE 20- 25 The collagen triple helix. (a) Left: Side view of the crystal structure of a polypeptide fragment whose sequence is based on repeating sets of three amino acids, Gly- X- Y, characteristic of collagen α chains. Center: Each chain is twisted into a left- handed helix, and three chains wrap around one another to form a right- handed triple helix. The schematic model (right) clearly illustrates the triple- helical nature of the structure and shows the left- handed twist of the individual collagen α chains (red line). (b) View down the axis of the triple helix. The proton side chains of the glycine residues (orange) point into the very narrow space between the polypeptide chains in the center of the triple helix. In collagen mutations in which other amino acids replace glycine, the proton in glycine is replaced by larger groups that disrupt the packing of the chains and destabilize the triple- helical structure. Data from R. Z. Kramer et al., 2001, J. Mol. Biol. 311:131, PDB ID 1bkv.
[Leave] [Close]