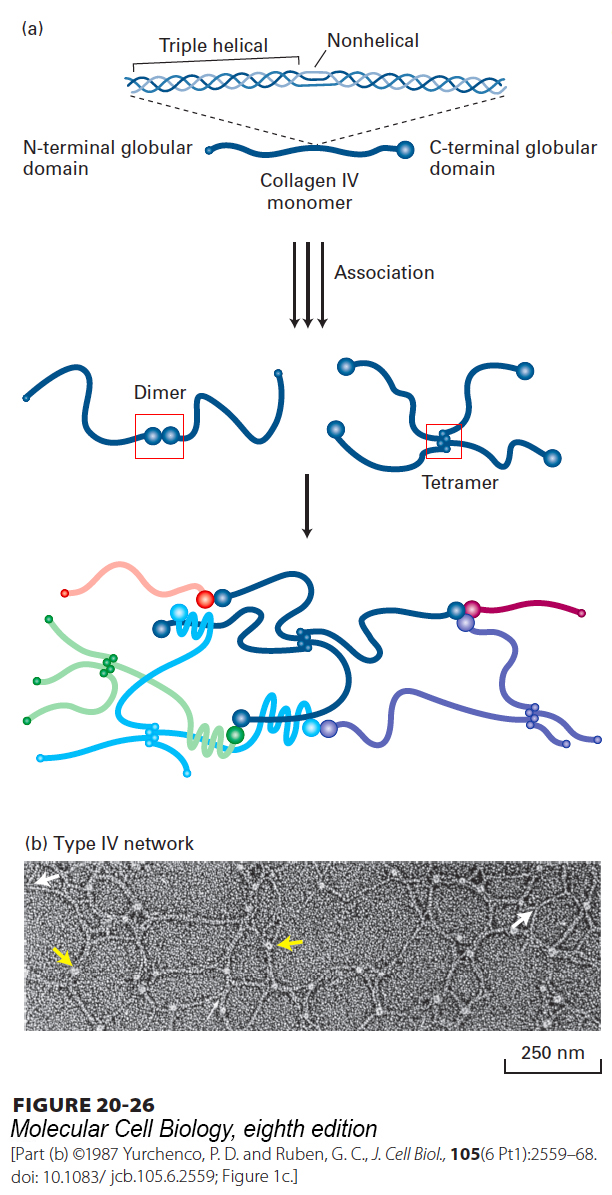
FIGURE 20- 26 Structure and assembly of type IV collagen. (a) Schematic representation of type IV collagen. This 400- nm- long molecule has a small noncollagenous globular domain at the N- terminus and a large globular domain at the C- terminus. The collagenous triple helix is interrupted by nonhelical segments that introduce flexible kinks into the molecule. Lateral interactions between triple- helical segments, as well as head- to- head and tail- to- tail interactions between the globular domains, form dimers, tetramers, and higher- order complexes, yielding a sheet- like network. Multiple, unusual sulfilimine (–S=N– ) or thioether bonds between hydroxylysine (or lysine) and methionine residues covalently cross- link some adjacent C- terminal domains and contribute to the stability of the network. See A. Boutaud, 2000, J. Biol. Chem. 275:30716. (b) Electron micrograph of type IV collagen network formed in vitro. The lacy appearance results from the flexibility of the molecule, the side- to- side binding between triple- helical segments (white arrows), and the interactions between C- terminal globular domains (yellow arrows).
[Part (b) ©1987 Yurchenco, P. D. and Ruben, G. C., J. Cell Biol., 105(6 Pt1):2559– 68. doi: 10.1083/jcb.105.6.2559; Figure 1c.]
[Leave] [Close]