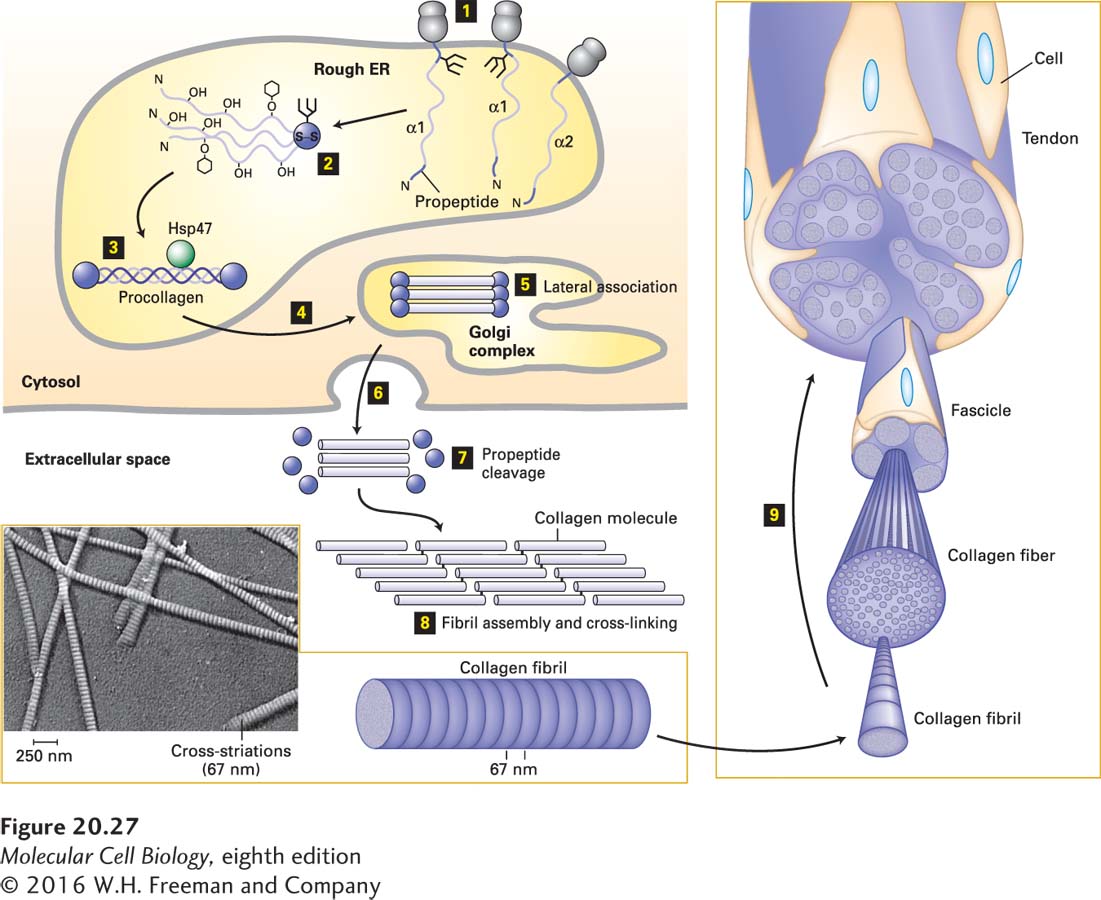
FIGURE 20- 27 Biosynthesis of fibrillar collagens. Step 1: Procollagen α chains are synthesized on ribosomes associated with the endoplasmic reticulum (rough ER), and in the ER, asparagine- linked oligosaccharides are added to the C- terminal propeptide. Step 2: Propeptides associate to form trimers and are covalently linked by disulfide bonds, and selected residues in the Gly- X- Y triplet repeats are covalently modified [certain prolines and lysines are hydroxylated, galactose or galactose- glucose (hexagons) are attached to some hydroxylysines, prolines are cis → trans isomerized]. Step 3: The modifications facilitate zipper- like formation and stabilization of triple helices, and binding by the chaperone protein Hsp47, which may stabilize the helices or prevent premature aggregation of the trimers, or both. Steps 4 and 5: The folded procollagens are transported to and through the Golgi complex, where some lateral association into small bundles takes place. The chains are then secreted (step 6), the N- and C- terminal propeptides are removed (step 7), and the trimers assemble into fibrils and are covalently cross- linked (step 8). The 67- nm staggering of the trimers gives the fibrils a striated appearance in electron micrographs (inset). Step 9: The fibrils can assemble into larger and larger bundles, some of which form the tendons that attach muscle to bone. See A. V. Persikov and B. Brodsky, 2002, Proc. Natl. Acad. Sci. USA 99:1101– 1103.
[Inset: Republished by permission of John Wiley & Sons, Inc., from Gross, J., “Evaluation of structural and chemical changes in connective tissue,” Ann. NY Acad. Sci., 1953, 56(4):674– 83; permission conveyed through the Copyright Clearance Center, Inc.]
[Leave] [Close]