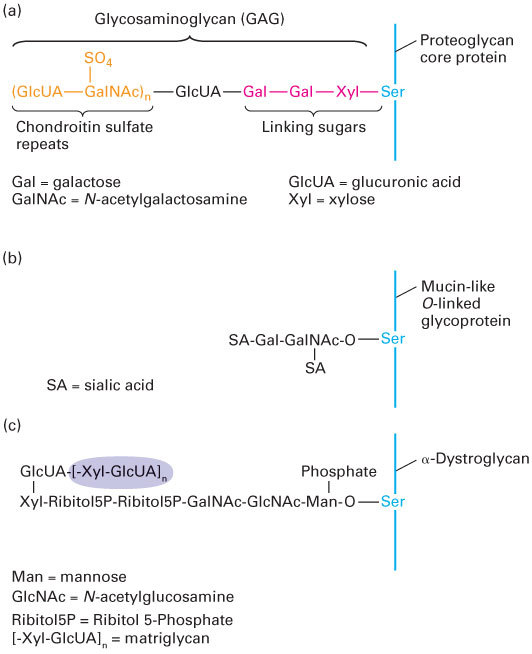
FIGURE 20- 30 Hydroxyl (O-) linked oligosaccharides. (a) Synthesis of a glycosaminoglycan (GAG), in this case chondroitin sulfate, is initiated by transfer of a xylose (Xyl) residue to a serine residue in the core protein, most likely in the Golgi complex, followed by sequential addition of two galactose (Gal) residues. Glucuronic acid (GlcUA) and N-acetylgalactosamine (GalNAc) residues are then added sequentially to these linking sugars and some of the GalNAc monomers are sulfated, forming the chondroitin sulfate chain. Heparan sulfate chains are connected to core proteins by the same three- sugar linker. Keratan sulfate GAGs are covalently attached to proteins via N-linked rather than O-linked connections. (b) Mucin- like O-linked chains are covalently bound to glycoproteins via an N-acetylgalactosamine (GalNAc) monosaccharide to which are covalently attached a variety of other sugars, often including sialic acid (SA). (c) Certain specialized O-linked oligosaccharides, such as those found in the adhesion receptor dystroglycan, are bound to proteins via mannose (Man) monosaccharides. The attachment of matriglycan, a polymer of the GlcUA- Xyl disaccharide (shaded), to the mannose via a phosphate and an additional unknown linkage (?) provides a binding site on dystroglycan for ECM molecules, such as laminin and perlecan.
[Leave] [Close]