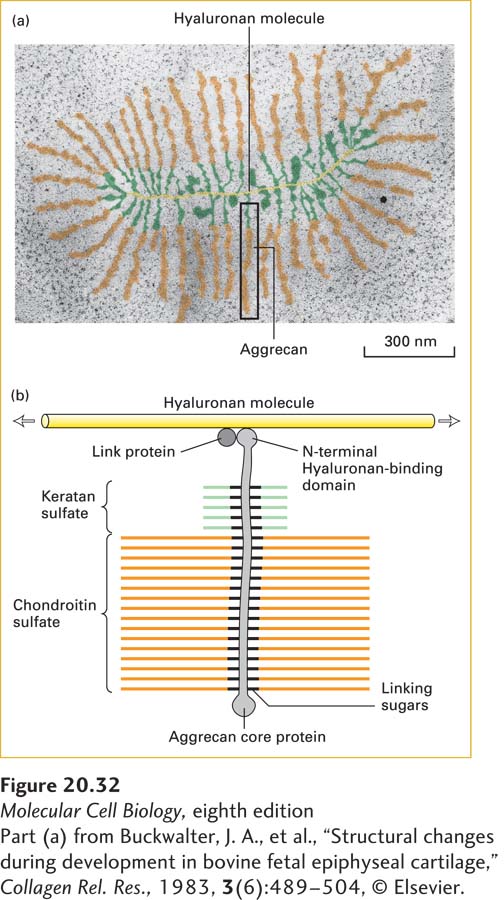
FIGURE 20- 32 Structure of proteoglycan aggregate from cartilage. (a) Electron micrograph of an aggrecan aggregate from fetal bovine epiphyseal cartilage. Aggrecan core proteins are bound at ~40- nm intervals to a molecule of hyaluronan. (b) Schematic representation of an aggrecan monomer bound to hyaluronan (yellow). In aggrecan, both keratan sulfate (green) and chondroitin sulfate (orange) chains are attached to the core protein. The N- terminal domain of the core protein binds noncovalently to a hyaluronan molecule. Binding is facilitated by a link protein, which binds to both the hyaluronan molecule and the aggrecan core protein. Each aggrecan core protein has 127 Ser- Gly sequences at which GAG chains can be added. The molecular weight of an aggrecan monomer averages 2 × 106. The entire aggregate, which may contain upward of 100 aggrecan monomers, has a molecular weight in excess of 2 × 108 and is about as large as the bacterium E. coli.
[Part (a) from Buckwalter, J. A., et al., “Structural changes during development in bovine fetal epiphyseal cartilage,” Collagen Rel. Res., 1983, 3(6):489– 504, © Elsevier.]
[Leave] [Close]