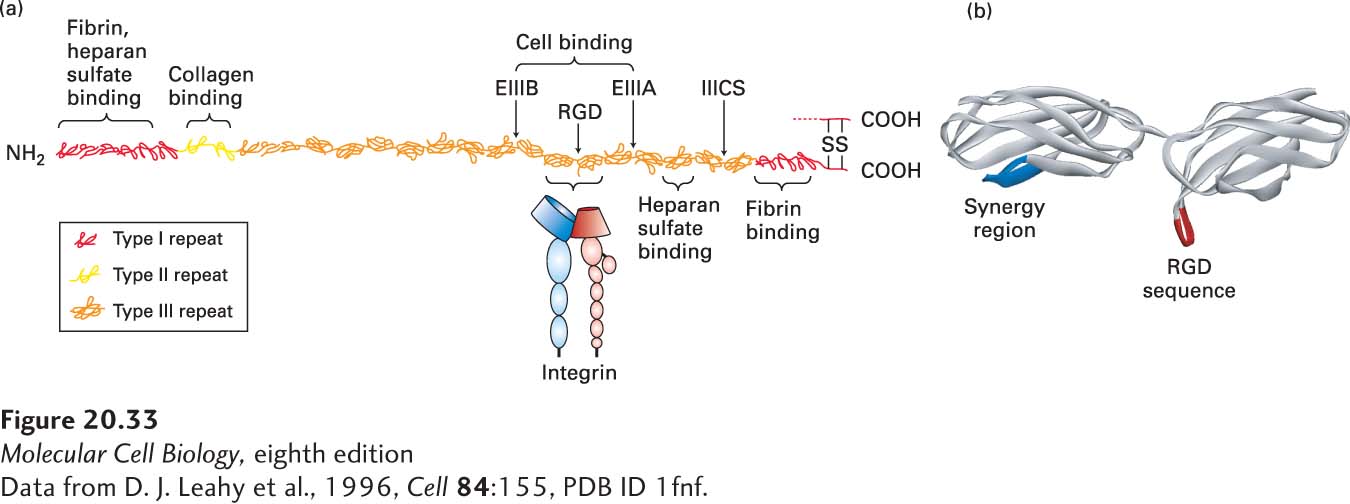
FIGURE 20- 33 Organization of fibronectin and its binding to integrin. (a) Scale model of fibronectin is shown docked by two type III repeats to the extracellular domains of integrin. Only one of the two similar chains, which are linked by disulfide bonds near their C- termini, in the dimeric fibronectin molecule is shown. Each chain contains about 2446 amino acids and is composed of three types of repeating amino acid sequences (type I, II, or III repeats) or domains. The EIIIA, EIIIB— both type III repeats— and IIICS domain are variably spliced into the structure at locations indicated by arrows. Circulating fibronectin lacks EIIIA, EIIIB, or both. At least five different sequences may be present in the IIICS region as a result of alternative splicing (see Figure 5- 16 ). Each chain contains several multi- repeat- containing regions, some of which contain specific binding sites for heparan sulfate, fibrin (a major constituent of blood clots), collagen, and cell- surface integrins. The integrin- binding domain is also known as the cell- binding domain. Structures of fibronectin’s domains were determined from fragments of the molecule. (b) A high- resolution structure shows that the RGD motif (red) extends outward in a loop from its compact type III domain on the same side of fibronectin as the synergy region (blue), which also contributes to high- affinity binding to integrins.
[Data from D. J. Leahy et al., 1996, Cell 84:155, PDB ID 1fnf.]
[Leave] [Close]