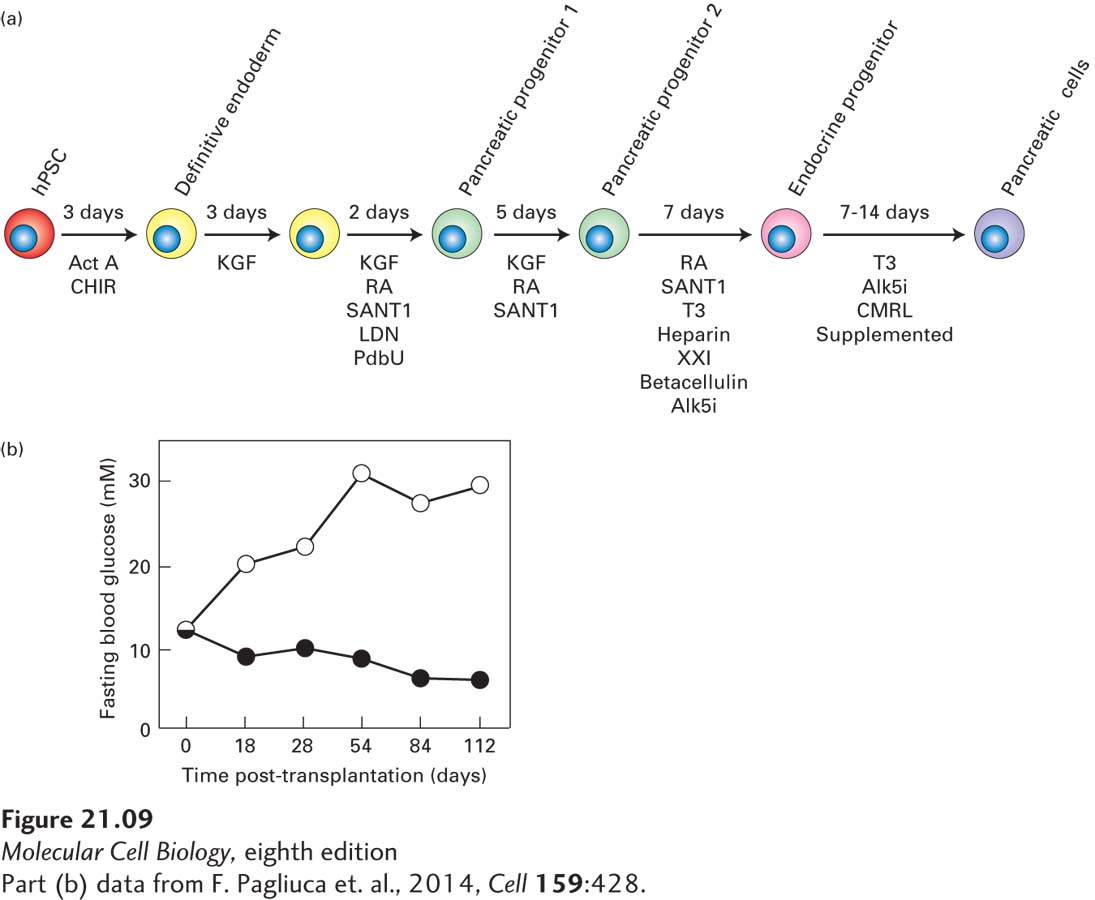
FIGURE 21- 9 Production of normal insulin- secreting β islet cells from human iPS or ES cells. (a) Schematic of directed differentiation of human ES or iPS cells into insulin- secreting β islet cells. Clusters of a few hundred human ES or iPS cells were sequentially cultured in media containing the indicated growth factors for the indicated number of days to first produce definitive endoderm cells, then a series of pancreatic progenitor cells, then pancreatic endocrine progenitors, and finally stem cell– derived insulin- producing β islet cells (termed SC- β cells). Act A, activin A; CHIR, GSK3 inhibitor; KGF, keratinocyte growth factor; RA, retinoic acid; SANT1, Sonic Hedgehog pathway antagonist; LDN, a BMP type 1 receptor inhibitor; PdbU, a protein kinase C activator; Alk5i, Alk5 receptor inhibitor II; T3, triiodothyronine, a thyroid hormone; XXI, γ-secretase inhibitor; betacellulin, an EGF family member. (b) SC- β cells can be used to treat diabetes in mice. These experiments used a strain of diabetic mice with a mutation in the insulin gene as well as mutations in several immune- system genes such that the animals did not reject transplants of human tissue. Previous work had shown that the elevated glucose levels in these mice could be restored to normal by transplantation with human pancreatic islets. In this experiment, mice were transplanted with SC- β cells (black circles) or a similar number of control pancreatic progenitor cells (white circles). At the start of the experiment, the average blood glucose level in these mice was about 11 mM, well above the normal 5 mM. The average blood glucose level in the control mice rose continuously to about 30 mM, indicating severe diabetes, while in the mice transplanted with the human SC- β cells, blood glucose dropped to nearly the normal 5 mM.
[Part (b) data from F. Pagliuca et al., 2014, Cell 159:428.]
[Leave] [Close]