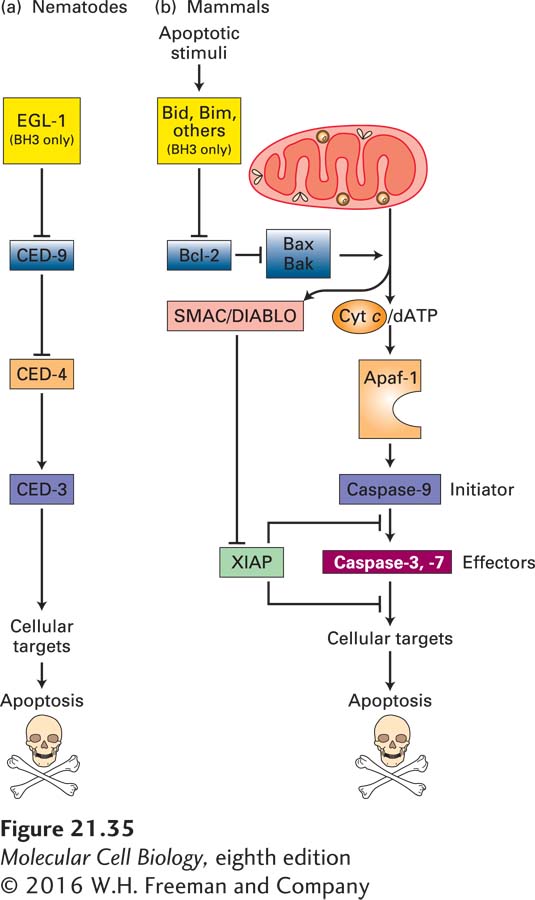
FIGURE 21- 35 Evolutionary conservation of apoptosis pathways. Similar proteins, shown in identical colors, play corresponding roles in nematodes and in mammals. (a) In nematodes, the BH3- only protein called EGL- 1 binds to CED- 9 on the outer mitochondrial membrane; this interaction releases CED- 4 from the CED- 4/CED- 9 complex. Free CED- 4 then binds to, and activates by autoproteolytic cleavage, the caspase CED- 3, which destroys cell proteins to drive apoptosis. These relationships are shown as a genetic pathway, with EGL- 1 inhibiting CED- 9, which in turn inhibits CED- 4. Active CED- 4 activates CED- 3. (b) In mammals, homologs of the nematode proteins, as well as many other proteins not found in the nematode, regulate apoptosis. The Bcl- 2 protein is similar to CED- 9 in promoting cell survival. It does so in part by preventing activation of Apaf- 1, which is similar to CED- 4, and in part by other mechanisms depicted in Figure 21- 40 . Several types of BH3- only proteins, detailed in Figures 21- 39 and 21- 40 , inhibit Bcl- 2 and thus allow apoptosis to proceed. Many apoptotic stimuli lead to damage of the outer mitochondrial membrane, causing release into the cytosol of several proteins that stimulate apoptosis. In particular, cytochrome c released from mitochondria activates Apaf- 1, which in turn activates caspase- 9. This initiator caspase then activates effector caspases- 3 and -7, eventually leading to apoptosis. See text for discussion of other mammalian proteins (SMAC/DIABLO and XIAP) that have no nematode homologs. See S. J. Riedl and Y. Shi, 2004, Nat. Rev. Mol. Cell Biol. 5:897.
[Leave] [Close]