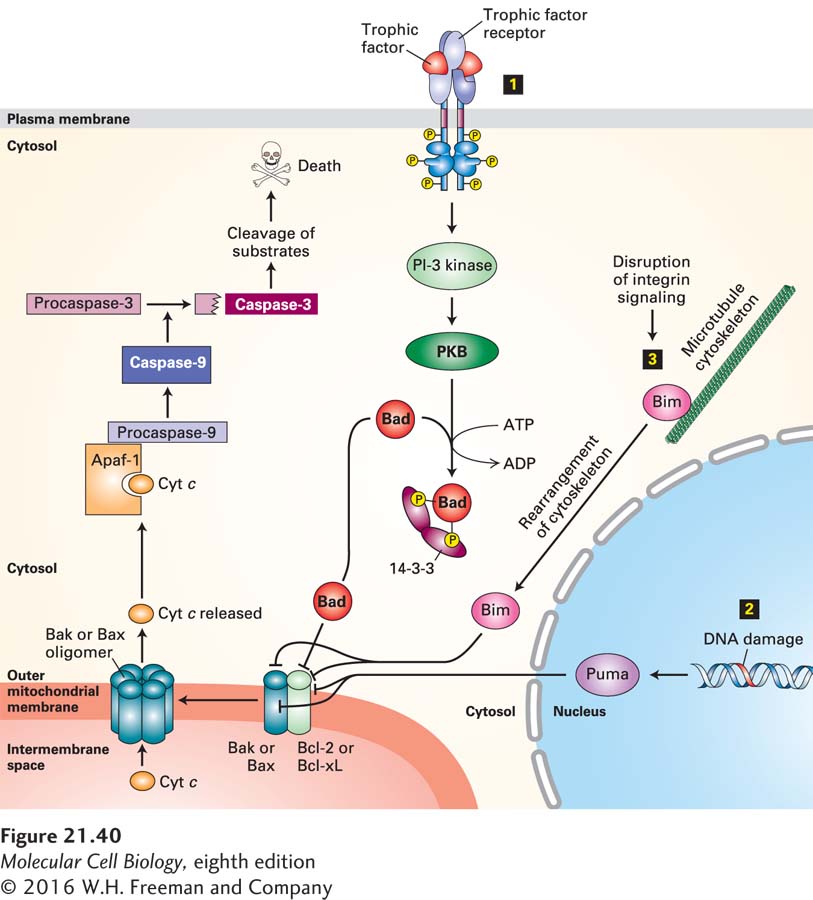
FIGURE 21- 40 Integration of multiple signaling pathways in vertebrate cells that regulate outer mitochondrial membrane permeability and apoptosis. In healthy cells, the anti- apoptotic protein Bcl- 2, or its homolog Bcl- xL, binds to Bak or Bax pro- apoptotic proteins, blocking the ability of Bak or Bax to oligomerize and form pores in the outer mitochondrial membrane. Binding of any of several BH3- only proteins, including Bad, Bim, and Puma, to Bcl- 2 or directly to Bak or Bax causes Bak or Bax to dissociate from Bcl- 2 and form oligomeric pores and holes in the outer mitochondrial membrane. These holes allow cytochrome c to enter the cytosol, where it binds to the adapter protein Apaf- 1, promoting caspase activation that initiates the apoptotic cascade and leads to cell death. Several stimuli trigger or repress this apoptotic pathway. Step 1 The presence of specific trophic factors (e.g., NGF) leads to activation of their cognate receptor tyrosine kinases (e.g., TrkA) and activation of the PI- 3 kinase– PKB (protein kinase B) pathway (see Figure 16- 29 ). PKB phosphorylates Bad, and phosphorylated Bad then forms a complex with a cytosolic 14- 3- 3 protein. This sequestered Bad is unable to bind to Bcl- 2. In the absence of trophic factors, nonphosphorylated Bad binds to Bcl- 2, releasing Bax and Bak and allowing them to form oligomeric membrane pores and holes. Step 2 DNA damage or ultraviolet irradiation leads to induction of synthesis of the BH3- only Puma protein. Puma binds to Bak and Bax as well as to Bcl- 2, allowing Bak and Bax to form oligomeric pores. Step 3 Removal of a cell from its substratum disrupts integrin signaling, leading to release of the BH3- only Bim protein from the cytoskeleton. Bim also binds to Bak and Bax to promote pore formation. See D. Ren et al., 2010, Science 330:1390 and Czabootar et al., 2014, Nat. Rev. Mol. Cell. Biol. 15:49.
[Leave] [Close]