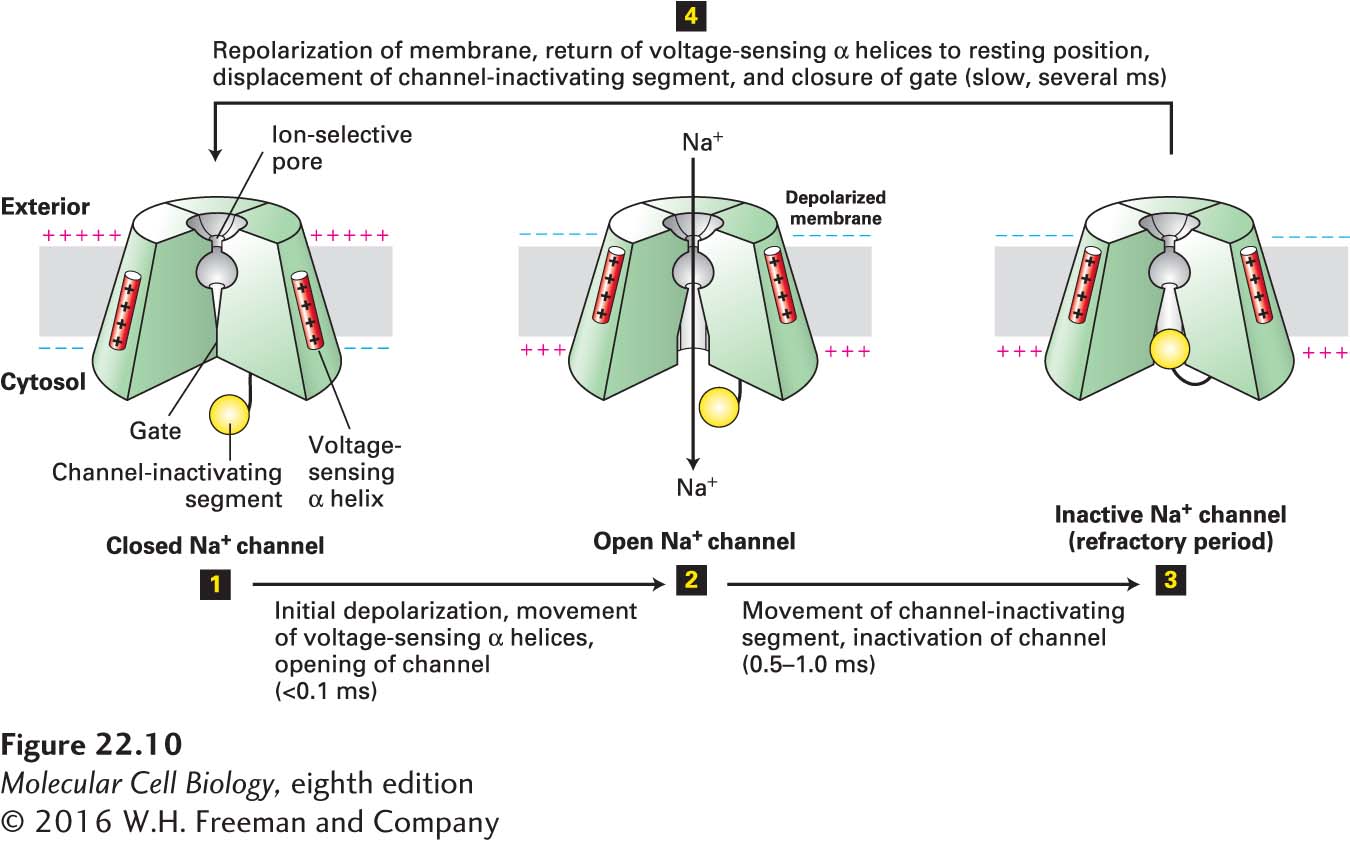
FIGURE 22- 10 Operational model of the voltage- gated Na + channel. As in the K+ channel depicted in Figure 11- 20 , four transmembrane domains in the protein contribute to the central pore through which ions move. The critical components that control movement of Na+ ions are shown here in the cutaway views depicting three of the four transmembrane domains. In the closed, resting state, the voltage- sensing α helices, which have positively charged side chains every third residue, are attracted to the negative charges on the cytosolic side of the resting membrane. This keeps the gate segment near the cytosolic face in a “closed” position that blocks the channel, preventing entry of Na+ ions (step 1). In response to a small depolarization, the voltage- sensing helices move through the phospholipid bilayer toward the outer membrane surface, causing an immediate conformational change in the gate at the cytosolic face of the protein that opens the channel (step 2). Within a fraction of a millisecond the channel- inactivating segment moves into the open channel, preventing passage of further ions (step 3). Once the membrane is repolarized, the voltage- sensing helices return to the resting position, the channel- inactivating segment is displaced from the channel opening, and the gate closes; the protein reverts to the closed, resting state and can be opened again by depolarization (step 4). See W. A. Catterall, 2001, Nature 409:988; and S. B. Long et al., 2007, Nature 450:376.
[Leave] [Close]