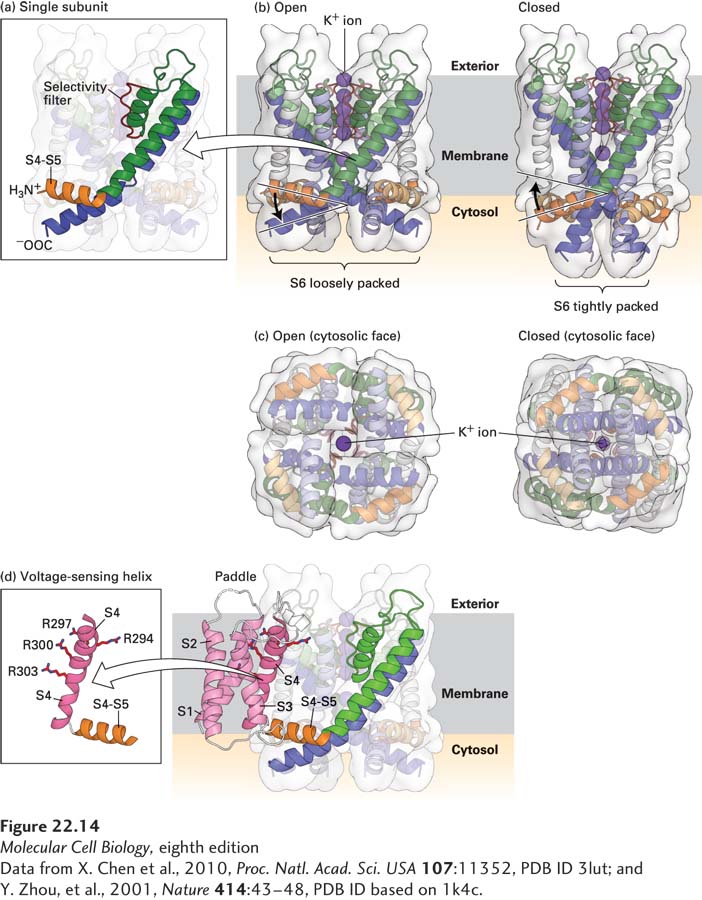
FIGURE 22- 14 Molecular structure of a voltage- sensitive K + channel. Models of the potassium channel single subunit (a) and tetramer (b) as viewed from the side, in open and closed states. The four green (S5) and blue (S6) α helices span the membrane, with the interior of the cell at the bottom and exterior at the top. Note how the helices are tightly packed at the bottom in the closed conformation, so that the K+ ion cannot pass through. (Compare the distances between S5 helices as shown by the curly brackets below (a) and (b).) The S4– S5 linker (orange), located in the cytoplasm, connects the S4 helix (not shown) to the S5 helix. For clarity, helices S1 through S4 have been omitted from the model; they would normally be attached to the end of the S4– S5 linker and protrude from the molecule. (c) Ribbon diagrams of the open and closed states of the channel as viewed from the cytoplasmic face of the membrane. In the open, but not in the closed, state, potassium ions (dark purple) can pass through the pore. (d) Three- dimensional structure of the voltage- sensing “paddles” comprising helixes S1– S4, with the four voltage- sensing arginine (R) residues in S4. These paddles move from near the interior to the exterior of the membrane in response to depolarization. Since each one is attached to an S4– S5 linker, each linker and its attached S5 helix is moved, in turn moving S6 helices, which opens the pore. Note that as shown in (b), the linker between S4 and S5 is pointed upward toward the exoplasmic (exterior) surface in the open channel, pulled upward by the outward movement of the S1– S4 paddles; in contrast the S4– S5 linker is pointing downward in the closed channel when the S1– S4 paddles are nearer the cytosolic surface.
[Data from X. Chen et al., 2010, Proc. Natl. Acad. Sci. USA 107:11352, PDB ID 3lut; and Y. Zhou, et al., 2001, Nature 414:43– 48, PDB ID based on 1k4c.]
[Leave] [Close]