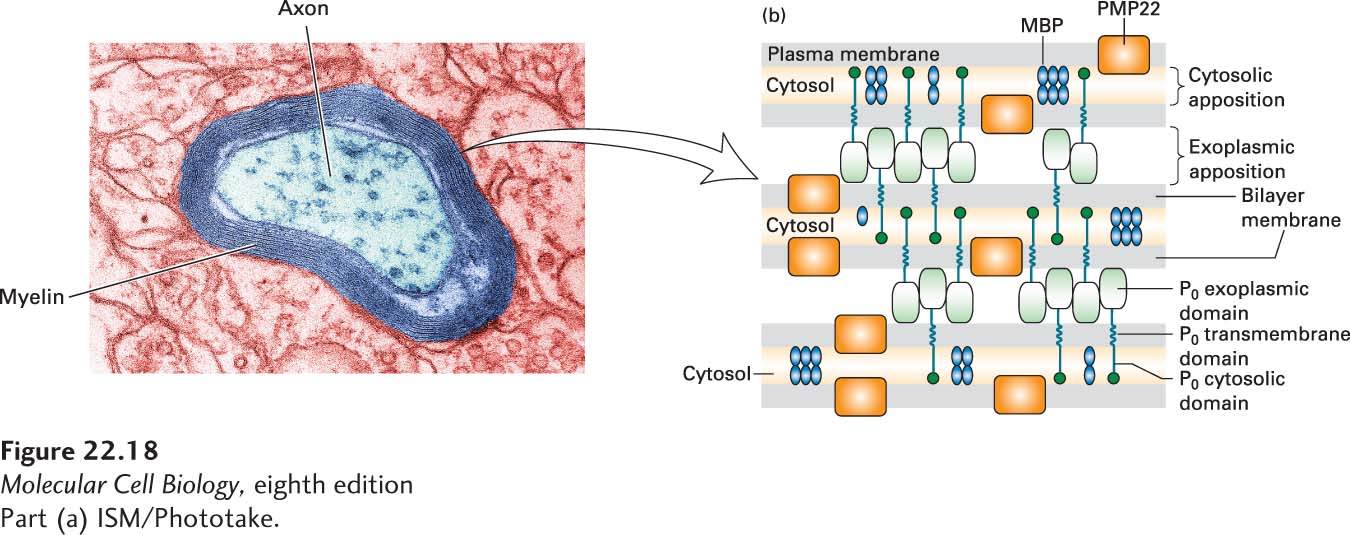
FIGURE 22- 18 Formation and structure of a myelin sheath in the peripheral nervous system. (a) At high magnification the specialized spiral myelin membrane appears as a series of layers, or lamellae, of phospholipid bilayers wrapped around the axon. (b) Close- up view of three layers of the myelin membrane spiral. The two most abundant integral myelin membrane proteins, P0 and PMP22, are produced only by Schwann cells. The exoplasmic domain of a P0 protein, which has an immunoglobulin fold, associates with similar domains emanating from P0 proteins in the opposite membrane surface, thereby “zippering” together the exoplasmic membrane surfaces in close apposition. These interactions are stabilized by binding of a tryptophan residue on the tip of the exoplasmic domain to lipids in the opposite membrane. Close apposition of the cytosolic faces of the membrane may result from binding of the cytosolic tail of each P0 protein to phospholipids in the opposite membrane. PMP22 may also contribute to membrane compaction. Myelin basic protein (MBP), a cytosolic protein, remains between the closely apposed membranes as the cytosol is squeezed out. See L. Shapiro et al., 1996, Neuron 17:435, and E. J. Arroyo and S. S. Scherer, 2000, Histochem. Cell Biol. 113:1.
[Part (a) ISM/Phototake.]
[Leave] [Close]