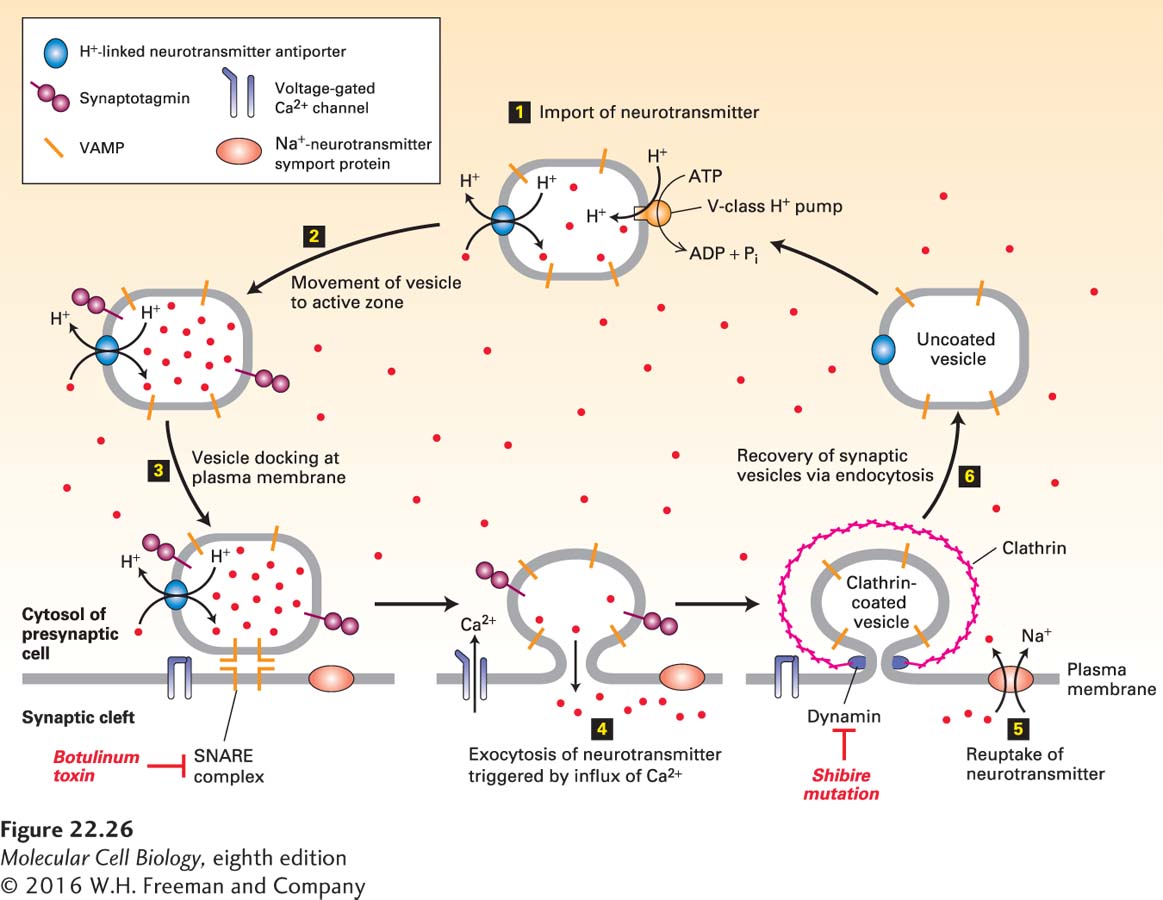
FIGURE 22- 26 Cycling of neurotransmitters and of synaptic vesicles in axon termini. Most synaptic vesicles are formed by endocytic recycling as depicted here. The entire cycle typically takes about 60 seconds. Step 1: The uncoated vesicles express a V- type proton pump (orange) and a single type of H+-neurotransmitter antiporter (blue) specific for the particular neurotransmitter, to import neurotransmitters (red dots) from the cytosol. Step 2: Synaptic vesicles loaded with neurotransmitter move to the active zone. Step 3: Vesicles dock at defined sites on the plasma membrane of the presynaptic cell, and the vesicle v- SNAREs called VAMP bind to the plasma membrane t- SNAREs, forming a SNARE complex. Synaptotagmin prevents membrane fusion and release of neurotransmitter. Botulinum toxin prevents exocytosis by proteolytically cleaving VAMP, the v- SNARE on vesicles. Step 4: In response to a nerve impulse (action potential), voltage- gated Ca2+ channels in the plasma membrane open, allowing an influx of Ca2+ from the extracellular medium. The resulting Ca2+-induced conformational change in synaptotagmin leads to fusion of docked vesicles with the plasma membrane and release of neurotransmitters into the synaptic cleft. Synaptotagmin does not participate in the later steps of vesicle recycling or neurotransmitter import though it is still present. Step 5: Na+ symporter proteins take up neurotransmitter from the synaptic cleft into the cytosol, which limits the duration of the action potential and partially recharges the cell with transmitter. Step 6: Vesicles are recovered by endocytosis, creating uncoated vesicles, ready to be refilled and begin the cycle anew. After clathrin/AP vesicles containing v- SNARE and neurotransmitter transporter proteins bud inward and are pinched off in a dynamin- mediated process, they lose their coat proteins. Dynamin mutations such as shibire in Drosophila block the re- formation of synaptic vesicles, leading to paralysis. Unlike most neurotransmitters, acetylcholine is not recycled. See K. Takei et al., 1996, J. Cell Biol. 133:1237; V. Murthy and C. Stevens, 1998, Nature 392:497; and R. Jahn et al., 2003, Cell 112:519.
[Leave] [Close]