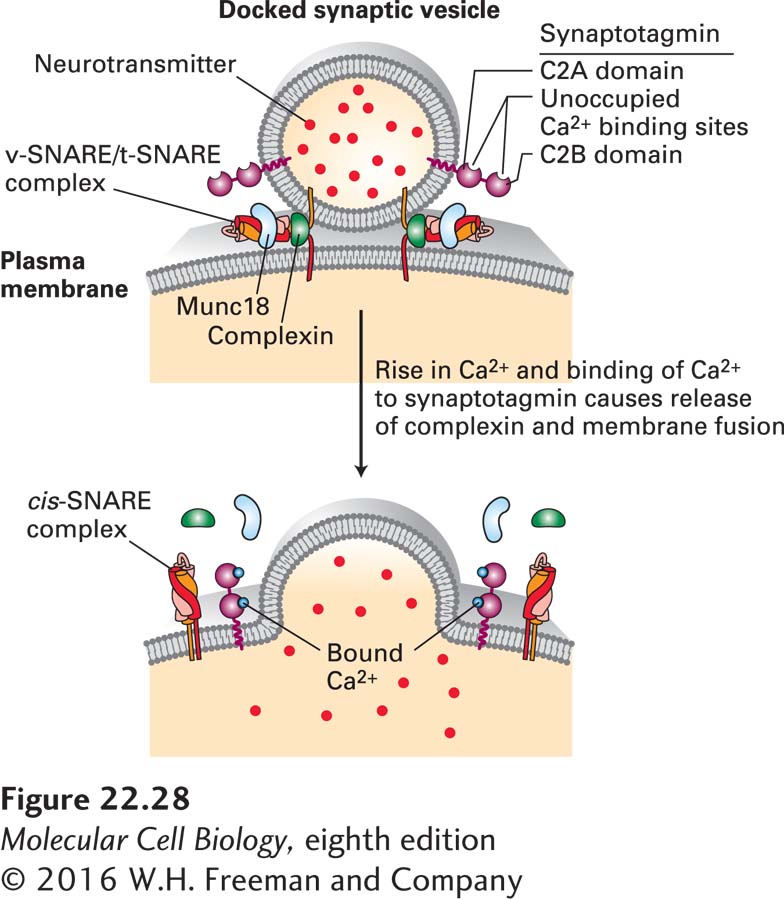
FIGURE 22- 28 Synaptotagmin- mediated fusion of synaptic vesicles with the plasma membrane. Only a few synaptic vesicles are docked at the presynaptic plasma membrane; these are primed for fusion with the plasma membrane. The tight interconnections between the synaptic vesicle and plasma membrane are mediated in part by bundles of four α helices derived from complexes of vesicle v- SNARE and plasma membrane t- SNARE proteins (see Figure 14- 10 ). The fusion of the two membranes is prevented by binding of complexin protein to the v- SNARE/t- SNARE complex. Synaptotagmin is composed of a short intraluminal sequence, a single transmembrane α helix that anchors it in the synaptic vesicle membrane, a linker, and two Ca2+-binding domains termed C2A and C2B. Synaptotagmin without bound Ca2+ may also bind to the v- SNARE/t- SNARE complex and prevent membrane fusion. A localized rise in Ca2+ allows Ca2+ ions to bind to synaptotagmin, altering its three- dimensional conformation. This triggers release of the complexin fusion inhibitor, binding (or altered binding) of synaptotagmin to the v- SNARE/t- SNARE complex, instantaneous membrane fusion, and release of neurotransmitters into the extracellular space. The SM protein Munc18, which binds to syntaxin, is required for SNARE- mediated fusion, although its precise mechanisms of action are not known. See T. Südhof and J. Rothman, 2009, Science 323:474 and T. Sudhof, 2013, Neuron 80:675– 690.
[Leave] [Close]