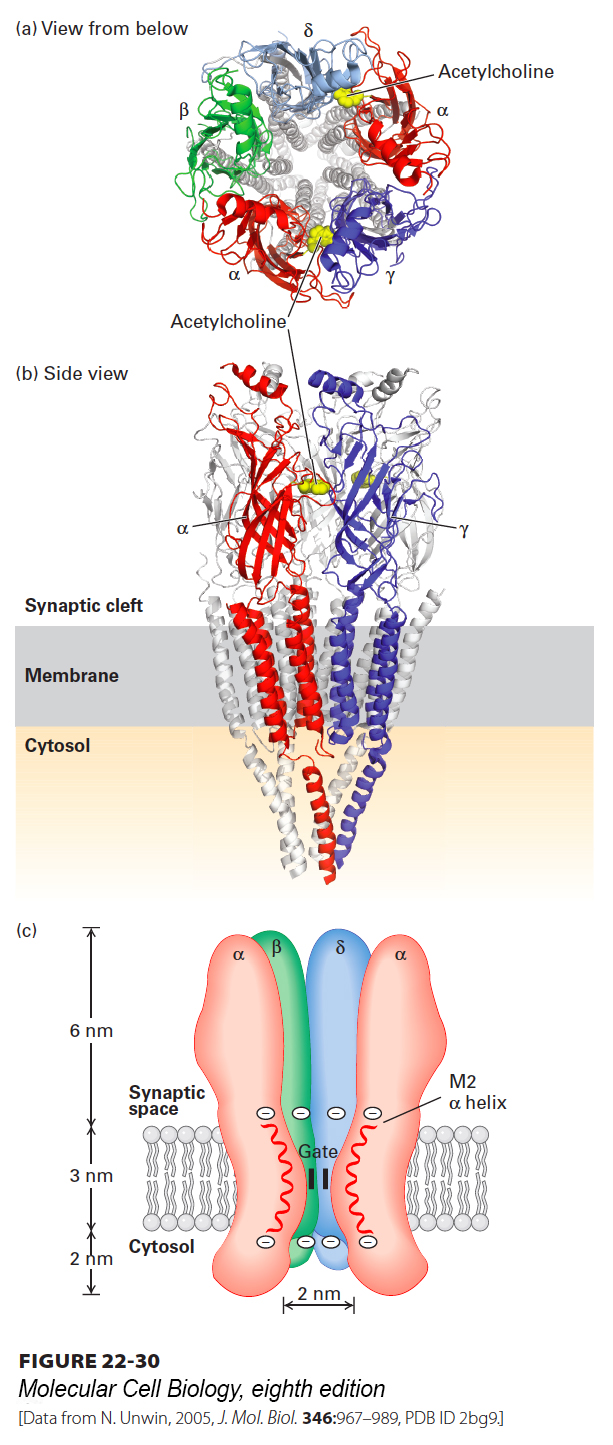
FIGURE 22- 30 Three- dimensional structure of the nicotinic acetylcholine receptor. Three- dimensional molecular structure of the Torpedo nicotinic acetylcholine receptor as viewed (a) from the synaptic cleft and (b) parallel to the plane of the membrane. For clarity, only the front two subunits, α and γ, are highlighted in (b) (colors: α, red; β, green; γ, blue; δ, light blue). The two acetylcholine- binding sites are located about 3 nm from the membrane surface and are highlighted in yellow; only the one at the α γ interface is shown in panel (b). (c) Schematic cutaway model of the pentameric receptor in the membrane. Each subunit has four membrane- spanning α helices, M1– M4; the M2 α helix (red) faces the central pore. Aspartate and glutamate side chains form two rings of negative charges, one at each end of the M2 helices, that help exclude anions from and attract cations to the channel. The gate, which is opened by binding of acetylcholine, lies within the pore.
[Data from N. Unwin, 2005, J. Mol. Biol. 346:967– 989, PDB ID 2bg9.]
[Leave] [Close]