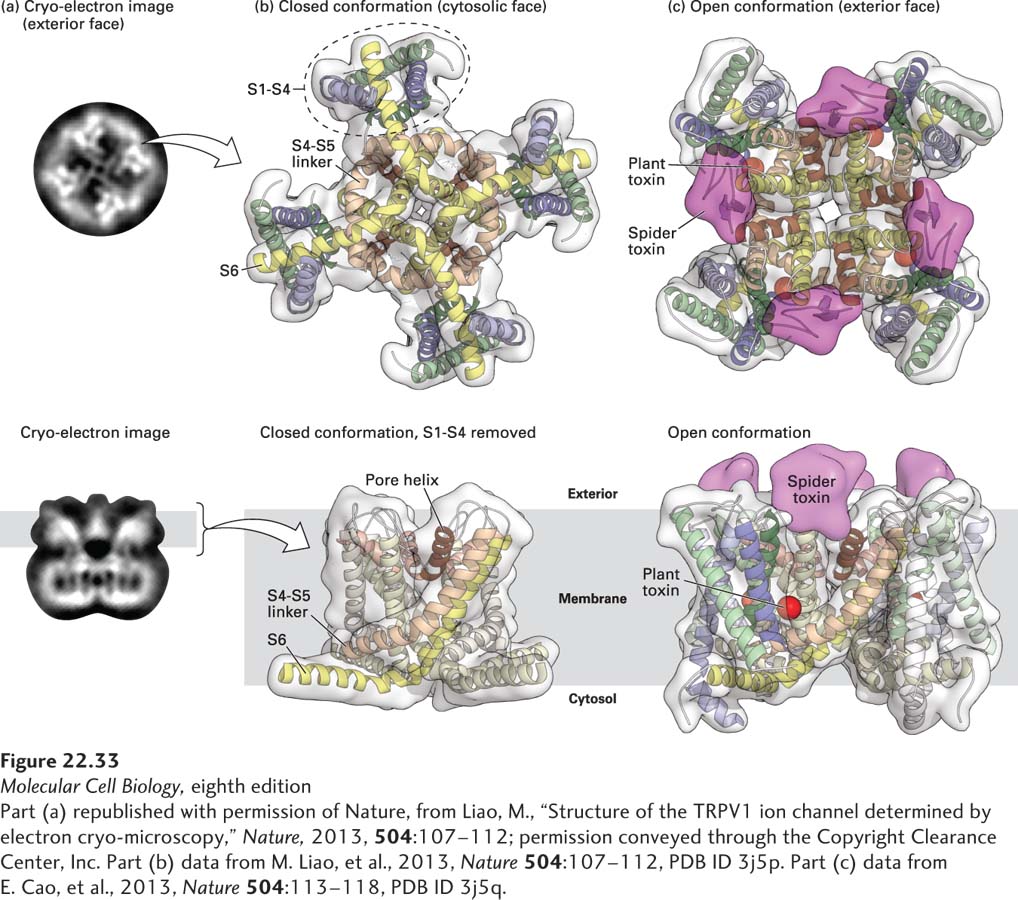
FIGURE 22- 33 Single- particle cyroelectron microscopy high- resolution structure of the TRPV1 channel. The high- resolution structure of the rat TRPV1 channel was obtained by single- particle cryoelectron microscopy at 0.34 nm resolution. (a) Photomicrographs of the two- dimensional structure of the tetrameric TRPV1 channel embedded in a thin layer of vitreous ice, with a face view of the channel in the top panel, and a side view in the bottom panel. (b, top) Ribbon diagram of a bottom view of the channel that focuses on the S1– S4 transmembrane domains, and the pore domain formed by S5 and S6, together with linking pore (P) loops. The S1– S4 domain is similar in structure to the voltage- sensing domains in the voltage- gated K+ and Na+ channels (see Figure 22- 14 ), but differ in that they do not move. (b, bottom) Ribbon diagram of the side view of the channel in the closed conformation, focusing on the pore domain that is formed by S5- P- S6. (c) The open conformation was stabilized by incubating the channel with two agonists, a spider toxin (in magenta) and a plant toxin (in red). Cryoelectron density maps reveal that the spider toxin (magenta) binds to external domains of the channel, linking two subunits of the channel together via its two globular cysteine- knot domains, while the plant toxin (red) binds to a region deep within the pore. Capsaicin binds to the same sites as the plant toxin (not shown). Binding of agonists to two distinct sites indicates that the TRPV1 channel is dually gated, allowing for significant modulation of channel function.
[Part (a) republished with permission of Nature, from Liao, M., “Structure of the TRPV1 ion channel determined by electron cryo- microscopy,” Nature, 2013, 504:107– 112; permission conveyed through the Copyright Clearance Center, Inc. Part (b) data from M. Liao, et al., 2013, Nature 504:107– 112, PDB ID 3j5p. Part (c) data from E. Cao, et al., 2013, Nature 504:113– 118, PDB ID 3j5q.]
[Leave] [Close]