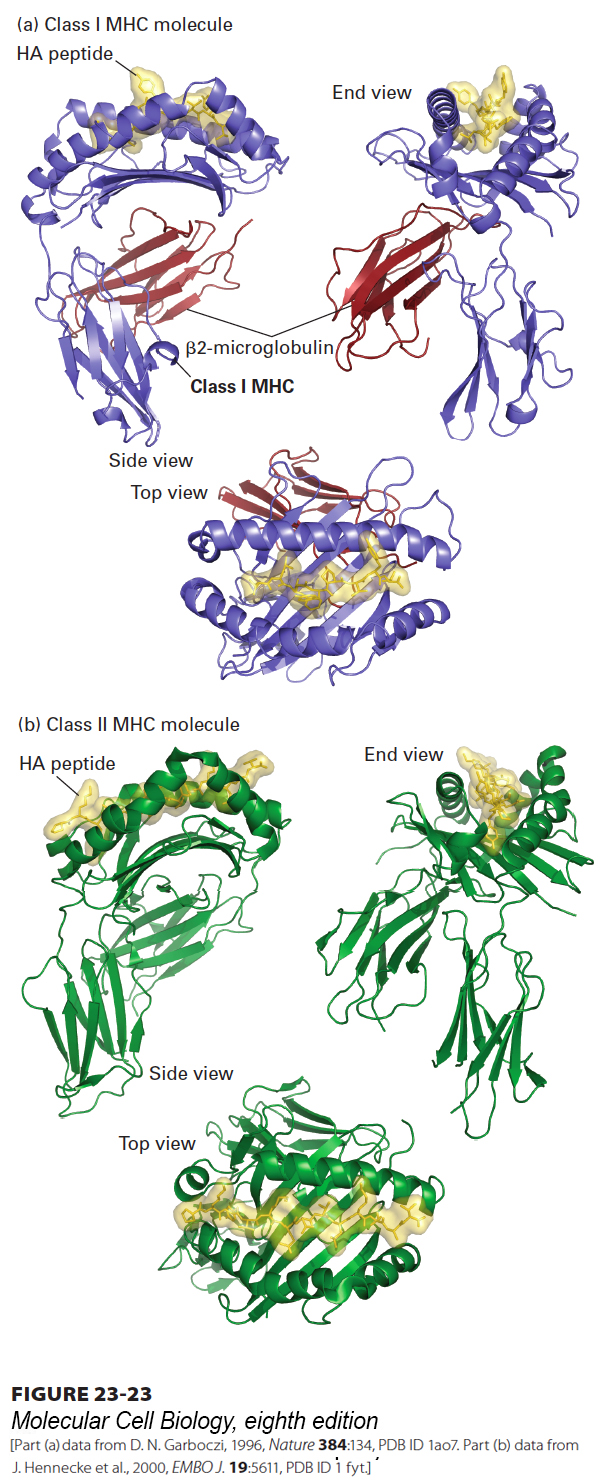
FIGURE 23- 23 Three- dimensional structure of class I and class II MHC molecules. (a) Shown here is the structure of a class I MHC molecule with bound antigenic (HA) peptide as determined by x- ray crystallography. The portion of a class I MHC molecule that binds a peptide consists of a β sheet composed of eight β strands and flanked by two α helices. The peptide- binding cleft is formed entirely from the MHC- encoded large subunit, which associates noncovalently with the small subunit (β2- microglobulin) encoded elsewhere. (b) Class II MHC molecules are structurally similar to class I molecules, but with several important distinctions. Both the α and β subunits of class II MHC molecules are MHC encoded and contribute to formation of the peptide- binding cleft. The peptide- binding cleft of class II MHC molecules accommodates a wider range of peptide sizes than that of class I molecules. The extracellular portions of class I and class II MHC products, both of which are type I membrane proteins, contain a transmembrane segment and a cytoplasmic tail (see Figures 23- 21 , 23- 26 , and 23- 29 ), not included in the crystallographic analysis.
[Part (a) data from D. N. Garboczi, 1996, Nature 384:134, PDB ID 1ao7. Part (b) data from J. Hennecke et al., 2000, EMBO J. 19:5611, PDB ID 1 fyt.]
[Leave] [Close]