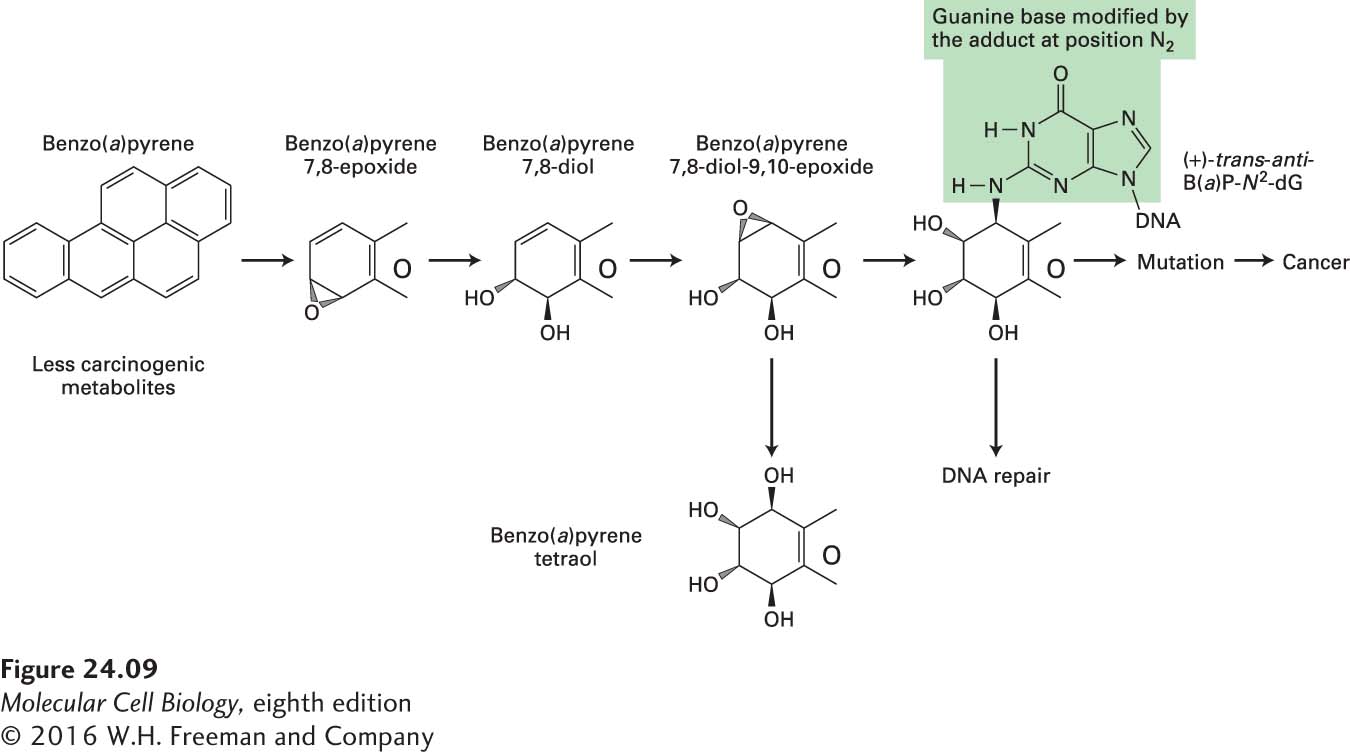
FIGURE 24- 9 Enzymatic processing of benzo(a)pyrene to a more potent mutagen and carcinogen. Liver enzymes, particularly P- 450 enzymes, modify benzo(a)pyrene in a series of reactions, producing 7,8- diol- 9,10- epoxide, a highly potent mutagenic species that reacts with DNA primarily at the N2 atom of a guanine base. The resulting adduct, (+)-trans- anti -B(a)P- N2-dG, causes polymerase to insert an A rather than a C opposite the modified G base. Next time the DNA is replicated, a T will be inserted opposite the A, and the mutation will be complete. Horizontal arrows indicate alterations toward greater potency, while vertical arrows indicate changes in the direction of reduced toxicity. The large “O” symbol represents the rest of the multi- ring structure shown in the complete benzo(a)pyrene molecule at the left.
[Leave] [Close]