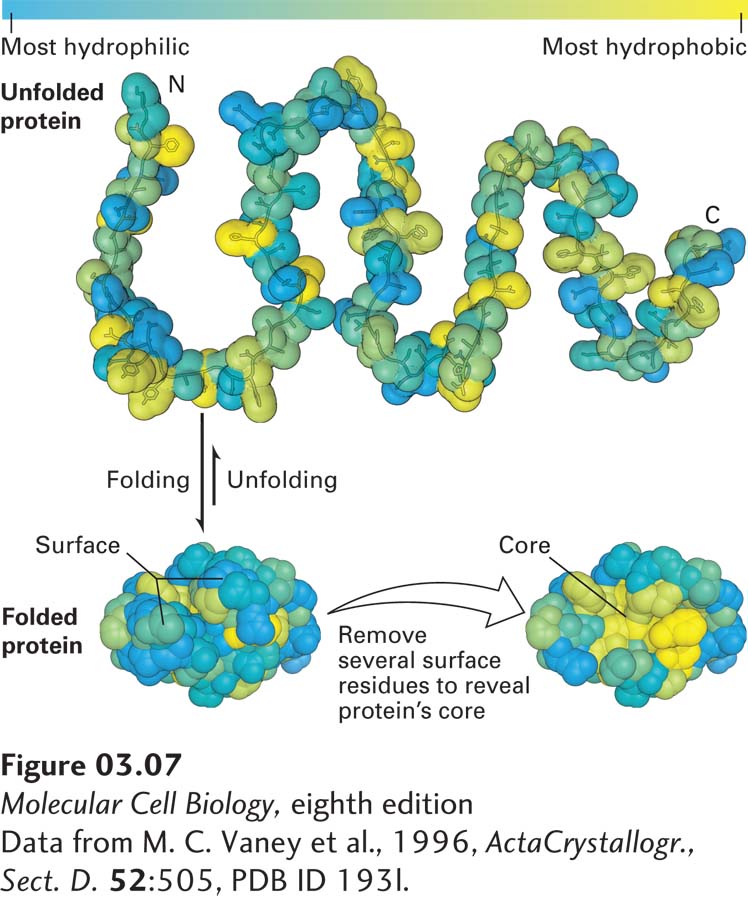
FIGURE 3- 7 The oil drop model of protein folding. The hydrophobic and hydrophilic residues of a polypeptide chain can be distributed throughout its linear sequence as illustrated in the unfolded protein (top). The color scale denotes the most most hydrophilic residues (blue) to the most hydrophobic (yellow). When the protein folds (bottom left), hydrophilic (charged and uncharged polar) side chains will often be exposed on the protein’s surface, where they can form stabilizing interactions with surrounding water and ions. In contrast, the hydrophobic residues tend to cluster together in the inner core, somewhat like drops of oil in an aqueous liquid, driven away from the aqueous surroundings by the hydrophobic effect (see Chapter 2). These core residues are more easily seen when several surface residues are removed (bottom right).
[Data from M. C. Vaney et al., 1996, Acta Crystallogr., Sect. D. 52:505, PDB ID 193l.]
[Leave] [Close]