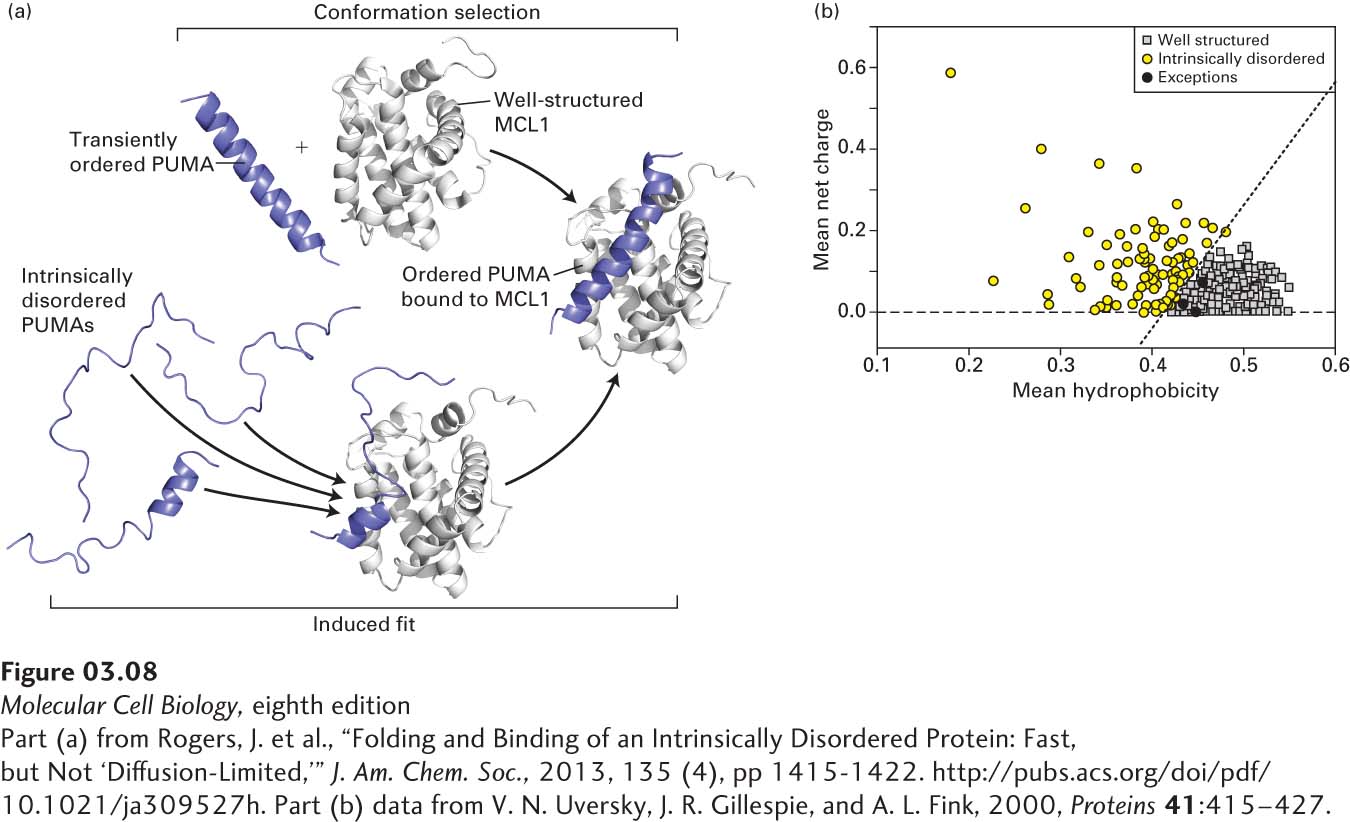
EXPERIMENTAL FIGURE 3- l- l- l- l- l- l- l- l- l-
[Part (a) from Rogers, J. et al., “Folding and Binding of an Intrinsically Disordered Protein: Fast, but Not ‘Diffusion- 5- 5–