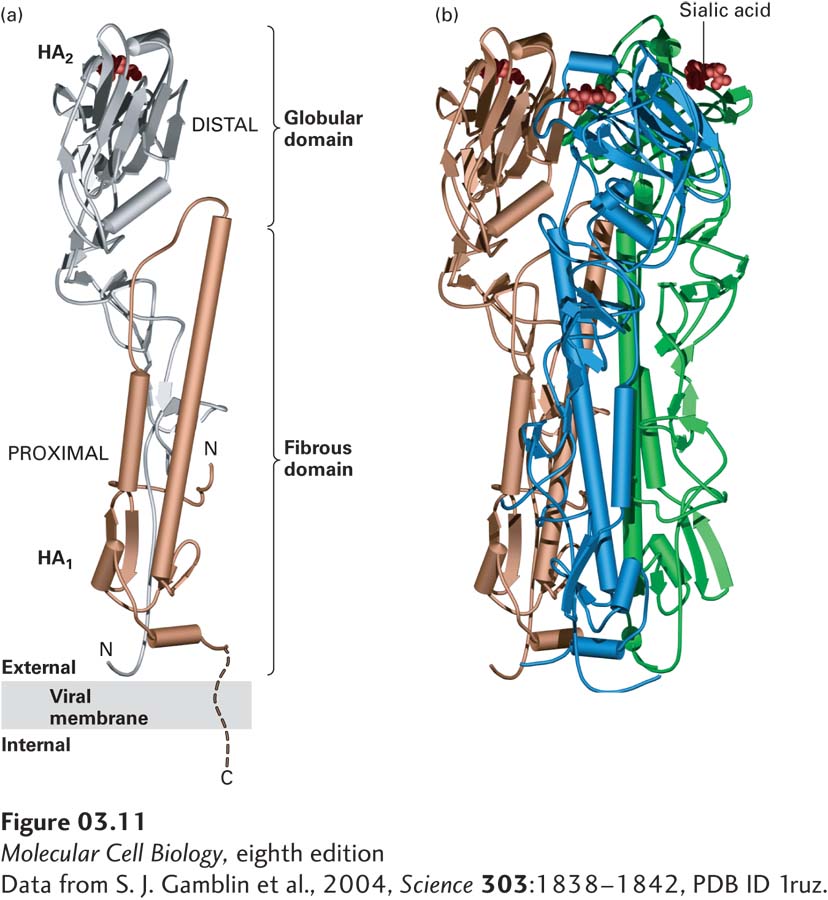
FIGURE 3- e- e- e- d-
[Data from S. J. Gamblin et al., 2004, Science 303:1838–