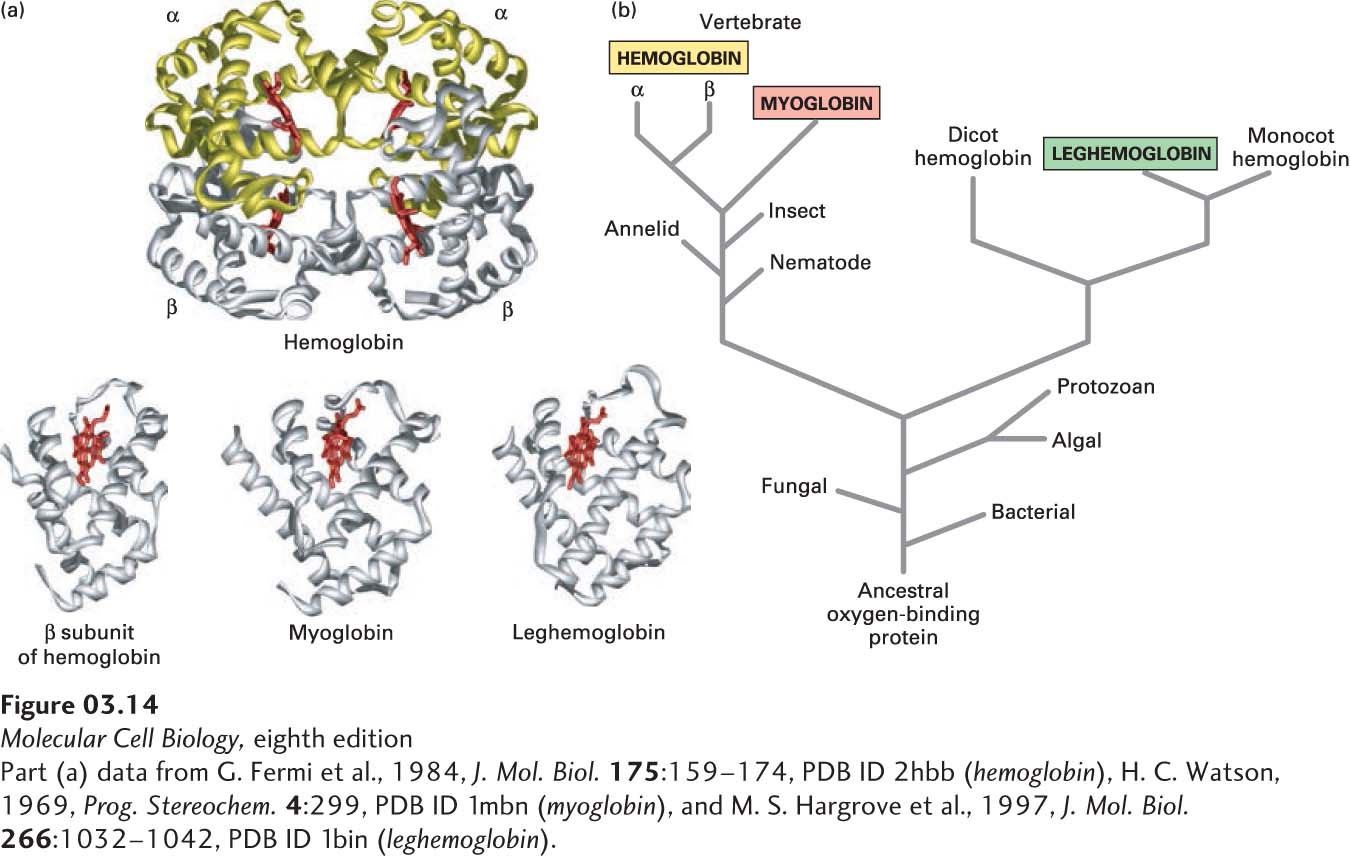
FIGURE 3- n- n-
[Part (a) data from G. Fermi et al., 1984, J. Mol. Biol. 175:159– 2–