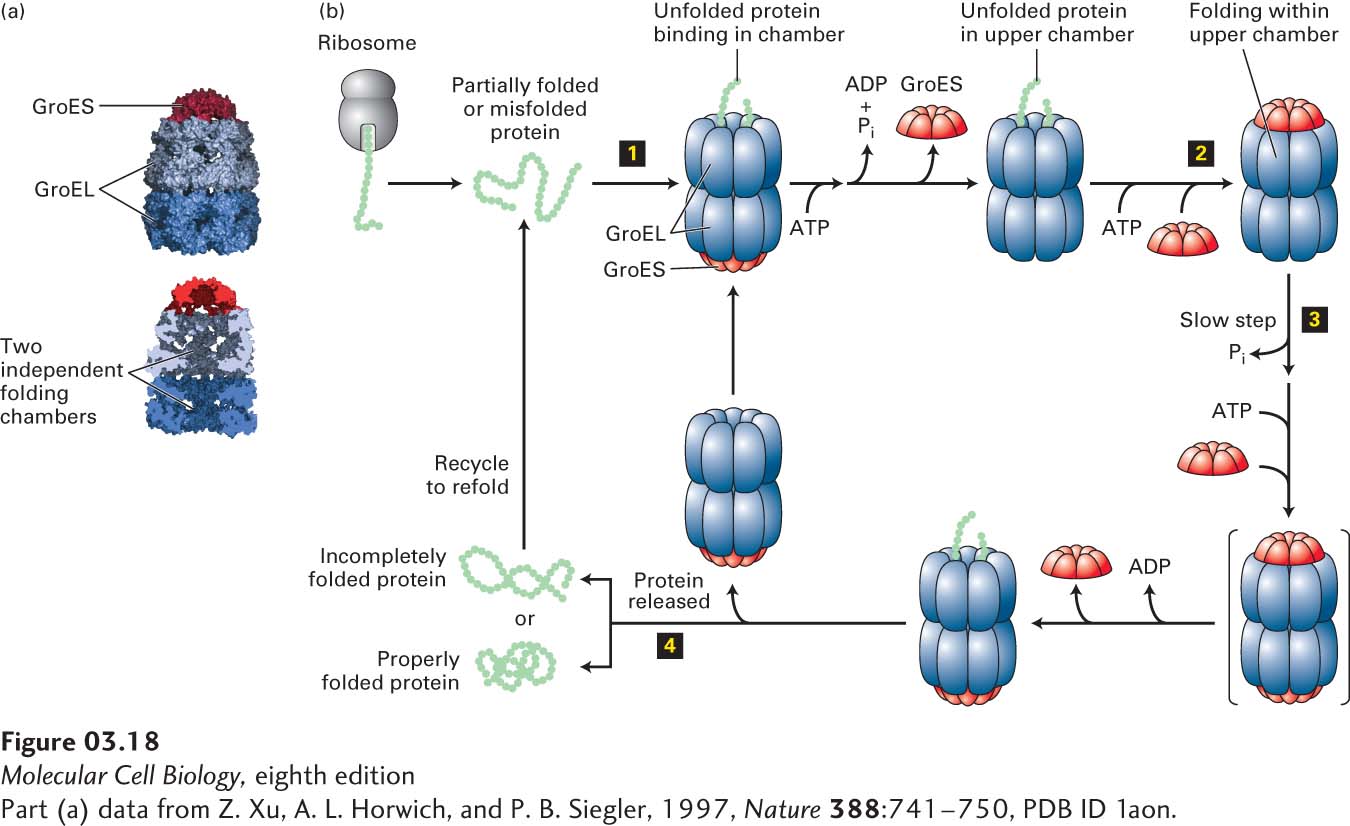
FIGURE 3- 18 Chaperonin- mediated protein folding. Proper folding of some proteins depends on chaperonins such as the prokaryotic group I chaperonin GroEL. (a) GroEL is a barrel- shaped complex of fourteen identical ~60,000- MW subunits, arranged in two stacked rings (blue) of seven subunits each that form two distinct internal polypeptide folding chambers. Homoheptameric lids (10,000- MW subunits), GroES (red), can bind to either end of the barrel and seal the chamber on that side. (b) The GroEL- GroES folding cycle. A partly folded or misfolded polypeptide enters one of the folding chambers (step 1). The second chamber is blocked by a GroES lid. Each ring of seven GroEL subunits binds seven ATPs, hydrolyzes them, and then releases the ADPs in a set order coordinated with GroES binding and release and polypeptide binding, folding, and release. The major conformational changes that take place in the GroEL rings control the binding of the GroES lid that seals the chamber (step 2). The polypeptide remains encased in the chamber capped by the lid, where it can undergo folding until ATP hydrolysis— the slowest, rate- limiting step in the cycle (t½ ~ 10 s) (step 3)—induces binding of ATP and a different GroES to the other ring (transient intermediate shown in brackets). This binding then causes the GroES lid and ADP bound to the peptide- containing ring to be released, opening the chamber and permitting the folded protein to diffuse out of the chamber (step 4). If the polypeptide has folded properly, it can proceed to function in the cell. If it remains partially folded or misfolded, it can rebind to an unoccupied GroEL and the cycle can be repeated. See D. L. Nelson and M. M. Cox, 2013, Lehninger Principles of Biochemistry, 6th ed., Macmillan.
[Part (a) data from Z. Xu, A. L. Horwich, and P. B. Siegler, 1997, Nature 388:741– 750, PDB ID 1aon.]
[Leave] [Close]