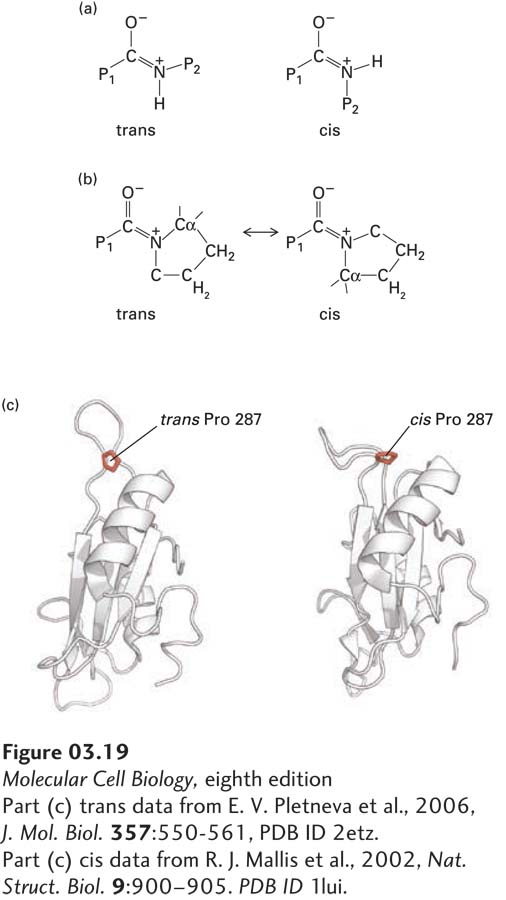
FIGURE 3- 19 Proline cis/trans isomerizations influence protein folding and structure. (a) The planar, double bond– like character of peptide bonds leads to the potential of the portions of the polypeptide chain on either side (P1 and P2) having cis or trans configurations. The trans configuration is present in about 99.97 percent of all peptide bonds in well- ordered proteins when P2 is a residue other than proline. (b) When P2 is proline, about 5 percent of peptide bonds are in the cis configuration. Proline isomerases catalyze the cis/trans isomerization to facilitate protein folding. (c) The structure of a portion of a protein, here an SH2 protein domain (see Chapter 16), can be dramatically altered by the cis/trans isomerization of a single proline, and this structural change can influence the protein’s activity.
[Part (c) trans data from E. V. Pletneva et al., 2006, J. Mol. Biol. 357:550- 561, PDB ID 2etz. Part (c) cis data from R. J. Mallis et al., 2002, Nat. Struct. Biol. 9:900– 905. PDB ID 1lui.]
[Leave] [Close]