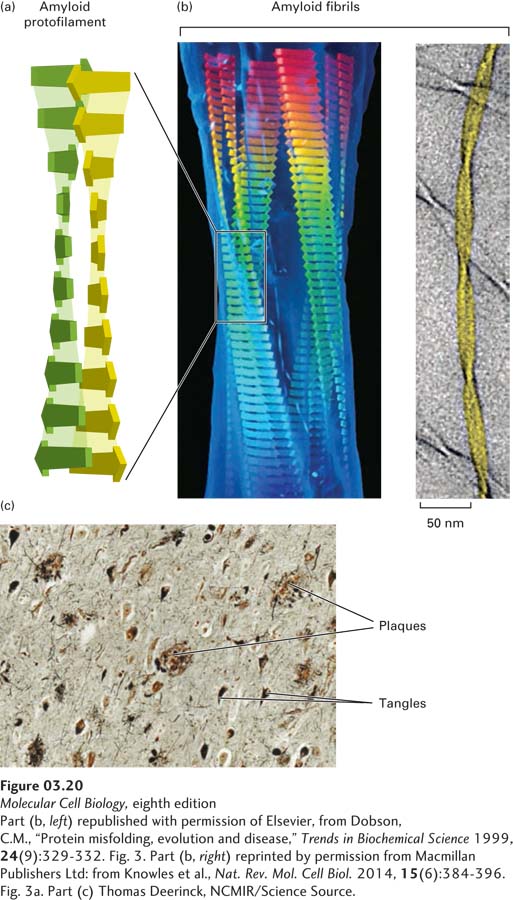
FIGURE 3- 20 Misfolded proteins can form ordered amyloid aggregates based on a cross- 6– 12 residues long (short flat arrows) can assemble into β sheets (see also Figure 3- 5 ) in which each β strand is oriented nearly perpendicularly to the long axis (vertical in this figure) of the resultant amyloid protofilament and hydrogen- bonded (light shading) to the strands above and below. Two long, nearly flat sheets pack closely together and twist around each other to form amyloid protofilaments, which then assemble together into thicker filaments called amyloid fibrils (b). Amyloid fibrils can be composed of varying numbers of protofilaments. A model of a four- protofilament- containing fibril fit into the electron density of acid- denatured insulin fibrils (left) and a cryoelectron microscopic image of two- protofilament- containing fibrils of fragments of transthyretin with an NMR- based model (yellow). Fibrils can aggregate into macroscopic plaques and tangles that are deposited in tissues and, when stained, are large enough to be visible using light microscopy. (c) Microscopic view of a section of human brain tissue from a patient with Alzheimer’s disease with multiple amyloid plaques and fibrillary tangles.
[Part (b, left) republished with permission of Elsevier, from Dobson, C.M., “Protein misfolding, evolution and disease,” Trends in Biochemical Science 1999, 24(9):329- 332. Fig. 3. Part (b, right) reprinted by permission from Macmillan Publishers Ltd: from Knowles et al., Nat. Rev. Mol. Cell Biol. 2014, 15(6):384- 396. Fig. 3a. Part (c) Thomas Deerinck, NCMIR/Science Source.]
[Leave] [Close]